EMRS PGT Chemistry Mock Test - 3 - EMRS MCQ
30 Questions MCQ Test - EMRS PGT Chemistry Mock Test - 3
Who is known as the founder of Modern Carnatic music?
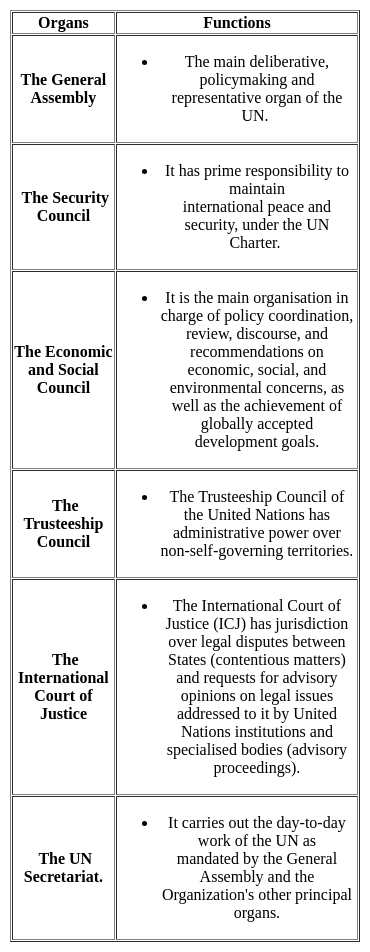
How many main organs are there in United Nations Organization?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
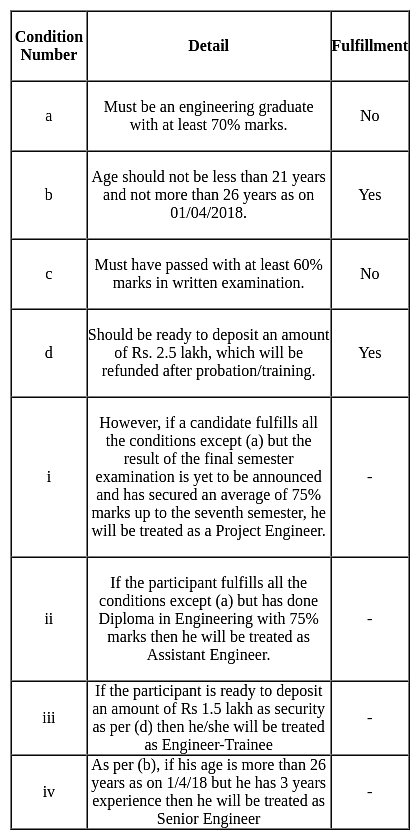
Rakul has completed Electrical Engineering with 69.5% marks. He has also passed the written exam with 59% marks. His age has become 22 years as on April 2018. He is ready to deposit Rs 2.5 lakh as security. For which position will he be recommended?
The major purpose of diagnostic test is that of identifying
Which of the following sentences is true about learning?
MnO4- + S2- + H+ → Mn2+ +S + H2O When this equation is balanced, the total number of coefficients on the left hand side are
Phenyl magnesium bromide reacts with methanol to give -
[AIEEE 2006]
In a mixture of A and B, components show negative deviation when [AIEEE-2002]
Which block of the periodic table contains the man made elements?
Enthalpies of formation of CO(g) , CO2 (g) , N2O (g) and N2O4 (g) are -110, - 393, 81 and 9.7 kJ mol-1. Thus, ΔrU for the reaction at 298 K is,
At 87°C, the following equilibrium is established
H2(g) + S(s) 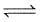 H2S(g) Kp = 7 × 10-2
H2S(g) Kp = 7 × 10-2
If 0.50 mole of hydrogen and 1.0 mole of sulfur are heated to 87°C in 1.0 L vessel, what will be the partial pressure of H2S at equilibrium ?
A resistance of 50Ω is registered when two electrodes are suspended into a beaker containing a dilute solution of a strong electrolyte such that exactly half of the them are submerged into solution. If the solution is diluted by adding pure water (negligible conductivity) so as to just completely submerge the electrodes, the new resistance offered by the solution would be
What’s the name of the 109th element as per the nomenclature?
A hydrocarbon X is optically. X upon hydrogenation gives an optically inactive alkane Y. Which of the following pair of compounds can be X and Y respectively?
Benzene and toluene form nearly ideal solutions. At 20ºC, the vapour pressure of benzene is 75 torr and that of toluene is 22 torr. The partial pressure of benzene at 20ºC for a solution containing 78 g of benzene and 46 g of toluene in torr is
[AIEEE-2005]
When a zinc rod is kept in a copper nitrate solution what happens?
"A young child responds to a new situation on the basis of the response made by him/ her in a similar situation as in the past". This is related to:
Hot conc. H2SO4 acts as moderately strong oxidising agent. It oxidises both metals and nonmetals. Which of the following element is oxidised by conc. H2SO4 into two gaseous products?
In the following question, out of the four alternatives, select the alternative which is the best substitute for the phrase.
Q. A situation that requires a lot of hard work.