EMRS PGT Chemistry Mock Test - 5 - EMRS MCQ
30 Questions MCQ Test - EMRS PGT Chemistry Mock Test - 5
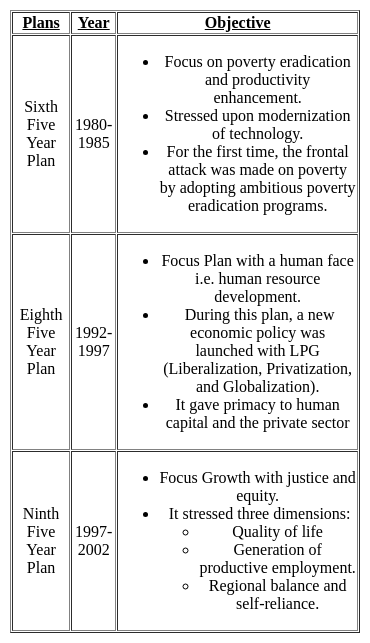
Arrange the following events of the French Revolution in chronological order and choose the right answer :
(a) Fall of Bastille
(b) Formation of National Assembly
(c) Establishment of the French Republic
(d) Declaration of the Rights of man
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
For first time Private sector was given priority compared to the public sector in.
Find the wrong number in the following number series:
121, 169, 289, 361, 529, 576
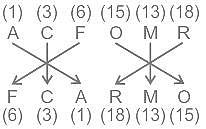
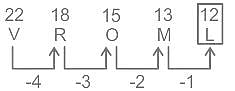
Select the related letters from the given alternatives.
ACFOMR : FCARMO :: MNTDEF : ?
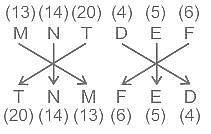
Choose the alternative which is an odd word/number/letter pair out of the given alternatives.
In which of the following process a software is used to create a mathematical model of some physical object as shown in the image?
Statement I : cis-2-butene with cold, dilute, alkaline KMnO4 gives meso-2,3- butanediol.
Statement II : In alkaline solution, under cold condition, KMnO4 acts as a mild oxidising agent.
The total number of tetrahedral voids in the face-centred unit cell is
Which of the following statement is true about Langmuir’s adsorption?
Based on the following thermodynamic data,
Q. On the total mass basis of reactants, which reaction will generate the greatest amount of heat?
Which compound is used as the cooling liquid in refrigerators?
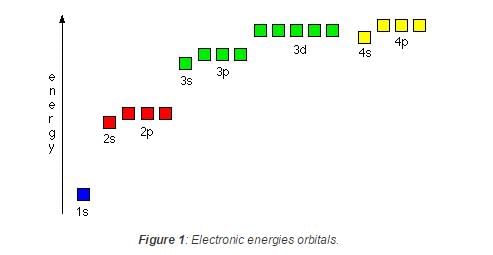
Which of the following sets of quantum numbers represents the highest energy of an atom ? [AIEEE 2007]
The increasing order of enthalpy of vaporization of NH3, PH3, and AsH3 is
PCl5 dissociation a closed container as :
PCl5(g) 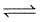 PCl3(g) + Cl2(g)
PCl3(g) + Cl2(g)
If total pressure at equilibrium of the reaction mixture is P and degree of dissociation of PCl5 is α, the partial pressure of PCl3 will be :
A metal in a compound can be displaced by another metal in the uncombined state. Which metal is a better reducing agent in such a case?
Which does not form dihalide when treated with ethylene glycol?
Which of the following is thermally the most stable?
Which of the following acids does not exhibit optical isomerism?
Direction (Q. Nos. 15-16) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d)
The complex [Fe(H2O)5NO]2+ is formed in the ring-test for nitrate ion when freshly prepared FeSO4 solution is added to aqueous solution of
followed by the addition of conc. H2SO4. NO exists as NO+ (nitrosyl).
Q.Oxidation number of the Fe in the ring is
What is the major product of the given reaction ?
Nucleoside differs from nucleotide with the absence of:
Determinants of individual differences in human beings relate to
Directions: Improve the bracketed part of the sentence.
Q. He (like) to picture himself as an original thinker.
Improve the bracketed part of the sentence with the parts given below.
Q. ONGC has claimed (to have start) a new updated natural gas refinery at Digboi.
दिए गए शब्द युग्म का सही अर्थ ज्ञात कीजिए।
सर्व-शर्व



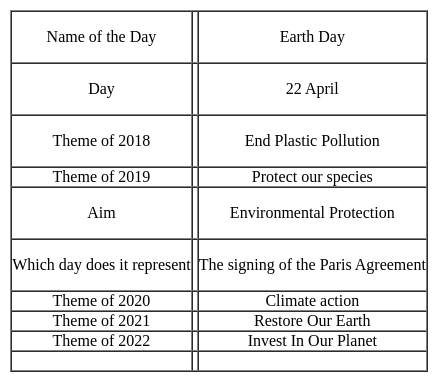

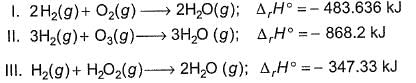 From I, total mass of reactant, 36 gm
From I, total mass of reactant, 36 gm