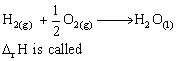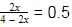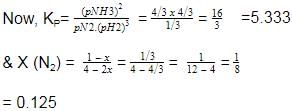EMRS PGT Chemistry Mock Test - 6 - EMRS MCQ
30 Questions MCQ Test - EMRS PGT Chemistry Mock Test - 6
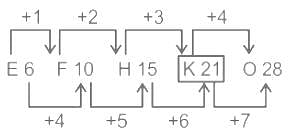
Find the missing term in the following series.
E6, F10, H15, ?, O28
Arrange the following words in the logical and meaningful order.
1. Designing
2. Manufacturing
3. Production
4. Planning
5. Implementation
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
A statement is given followed by two conclusions. Find which conclusion(s) is /are true based on the given statement.
Statements:
K > M < L = Z = O < P
Conclusions:
I. P < Z
II. M = Z
In a certain code, MONK is written as 53. How will TUTOR be written in that code?
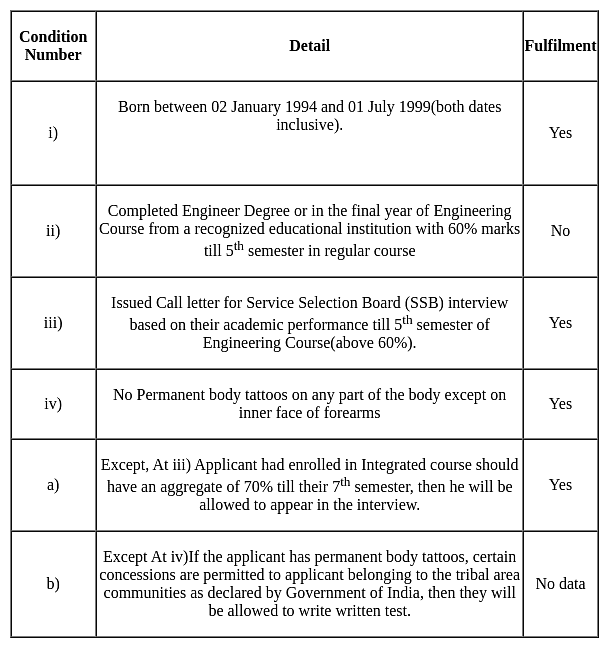
Jatin, born on 05 January 1998, is in final year of Engineering of an Integrated course, with an aggregate of 75% till his 7th semester, he does not have any permanent body tattoos.
Select the option which is related to the third term in the same way as the second term is related to the first term.
Synonym : Similar :: Opposite : ?
What is the shortcut key to create a new document in MS-Word?
Which of the following elements are called representative elements?
Pyrolusite in MnO2 is used to prepare KMnO4. Steps are
Here, I and II are
The frequency of light emitted for the transition n = 4 to n = 2 of He+ is equal to the transition in H atom corresponding to which of the following
For the reaction,
2SO2 (g) + O2 (g)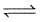 2SO3 (g) + 188.3 KJ
2SO3 (g) + 188.3 KJ
the number of moles of SO3 formed is increased if
Which of the following equation depicts reducing nature of H2O2?
At 700 K and 350 bar, a 1 : 3 mixture of N2(g) and H2(g) reacts to form an equilibrium mixture containing X (NH3)= 0.50. Assuming ideal behaviour Kp for the equilibrium reaction,
Direction (Q. Nos. 11-14) This section contains 2 paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d).
Passage l
Sulphur undergoes a phase transition between 80 and 110°C
S(rhombic) S (monoclinic); ΔH° = 3.213 kJ mol-1; ΔS° = 8.71 JK-1 mol-1
Q. Select the correct alternate(s).
A mixture of ethyl alcohol and propyl alcohol has a vapour pressure of 290 mm at 300 K. The vapour pressure of propyl alcohol is 200 mm. If the mole fraction of ethyl alcohol is 0.6, its vapour pressure (in mm) at the same temperature will be -
[AIEEE 2007]
The energies of activation for forward and reverse reactions for A2 + B2 2AB are 180 kJ mol–1 and 200 kJ mol–1 respectively. The presence of a catalyst lowers the activation energy of both (forward and reverse) reactions by 100 kJ mol–1. The enthalpy change of the reaction (A2 + B2 → 2AB) in the presence of catalyst will be (in kJ mol–1) –
[AIEEE 2007]
What are the basic constituent particles forming diamond crystals?
One or More than One Options Correct Type
This section contains 3 multiple type questions. Each question has 4 choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q.
Following solutions have been provided at temperature T K.
I. 1M aqueous glucose solution.
II. 1M aqueous sodium chloride solution.
III. 1M aqueous ammonium phosphate solution.
IV. 1M benzoic acid in benzene.
Select correct statements for the above solutions.
Direction (Q. Nos. 19-20) This section contains a paragraph, each describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d)
Valence shell MO electronic configuration of a diatomic species is shown
* is for anti-bonding molecular orbital (MO).
Q. Bond order of this species is
Consider the ground state of Cr atom (Z = 24). The numbers of electrons with the azimuthal quantum numbers, = 1 and 2 are, respectively:
The question below consists of a set of labeled sentences. Out of the four options given, select the most logical order of the sentences to form a coherent paragraph.
P: The climate has been changing drastically over the past years.
Q: If we don't correct the situation now, maybe we will never get a chance again.
R: Human activities itself are the main reason behind this.
S: The redressal of this problem is the biggest challenge of our generation.
Improve the bracketed part of the sentence with the parts given below.
Q. The police referred to him as (a complete cheat).