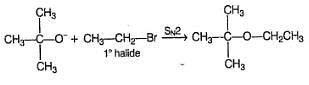NVS PGT Chemistry Mock Test - 2 - NVS TGT/PGT MCQ
30 Questions MCQ Test - NVS PGT Chemistry Mock Test - 2
When was the first meeting of Constituent Assembly held?
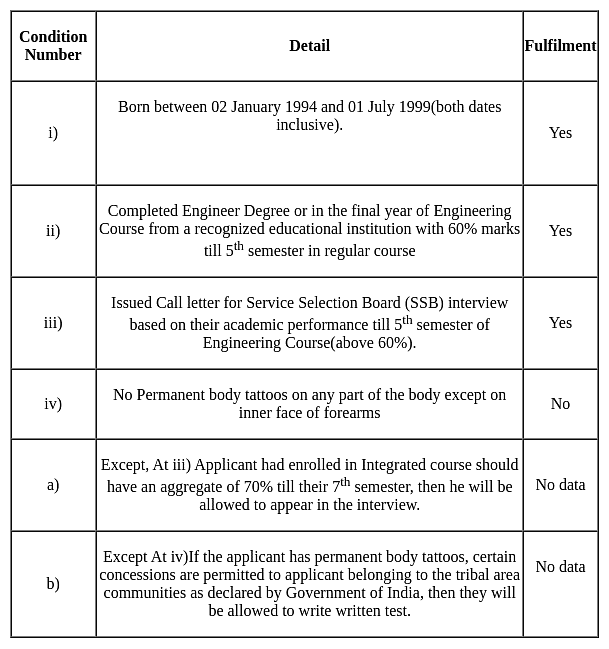
Vishnu born on 15 January 1995, completed his engineering degree, with 85% marks till 5th semester in regular course, he has a permanent body tattoo in his shoulder.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Find the word that can be suffixed to the given words to form new meaningful words.
Fail, Press, Depart, Advent
In an experiment conducted on a hungry chimpanzee, some bananas were kept outside its cage, but beyond its reach. Some sticks were also kept in its cage. After several unsuccessful attempts to reach out to the bananas, the chimpanzee pondered over the problem. Then, he picked up a stick and pulled the bananas towards itself. In this case, learning took place by
Madam Soni found that most of the students this year performed better in exams than the last year. This may be due to:
Which among the following does not exhibit positive oxidation state?
Which of the following are all properties of nonmetals?
Optical rotation of a newly synthesised chiral compound is found to be +60°. Which of the following experiment can be performed to establish that optical rotation is not actually -300°?
Comprehension Type
This section contains a passage describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer out of the given 4 options (a), (b), (c) and (d)
Passage I
Hgl2 is insoluble in water. This solute is added into Kl solution.
Q.
In the above case,
Which is the best reaction for preparation of t-butyl ethyl ether?
Given, ΔfH° of HCI (g) is - 22 . 10 kcal mol-1 and ΔSolutionH° (heat of solution) of HCI (g) is - 17.9 kcal mol-1. Thus, ΔfH ° of Cl- (aq) is
Which of following statement is incorrect regarding ionization enthalpy:
Consider the following set of molecules.
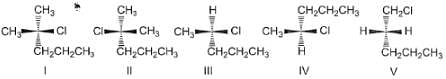
The pairs of enantiomers are
In the complete combustion of C2H6, 54 g of H2O is formed and 370 kcal of heat is evolved. Thus, ΔCH° of C2H6 is
The decreasing order of the repulsive interactions between various electron pairs is:
Which is the intramolecular oxidation-reduction reaction?
Which major organic product is formed in the following reaction ?
When sodium is dissolved in liquid ammonia, a solution of deep blue colour is obtained. The colour of the solution is due to
presence of extensive hydrogen bonding between water molecules leads to
Which has lower standard reduction potential (SRP) value?
The ≈ pH of the neutralisation point of 0.1 N ammonium hydroxide with 0.1 N HCl is
Directions: In the following question, out of the four alternatives, select the word opposite in meaning to the word given.
Castigate
The following sentence has been broken into four parts with an error in one part. Identify that part and mark it as your answer. If there are no errors in any of the given parts, mark option 4 or ‘No error’ as your answer.
Q. No news of firing (1)/ along the border (2)/ have been heard of. (3)/ No error (4).