NVS PGT Chemistry Mock Test - 3 - NVS TGT/PGT MCQ
30 Questions MCQ Test - NVS PGT Chemistry Mock Test - 3
Which of the following has the shortest wavelength?
Who determines the minimum support price in India?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The procedure of Impeachment of the President of India is __________.
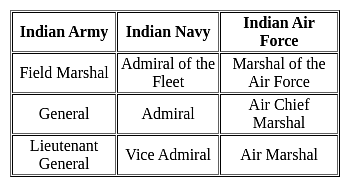
Select the correct option that indicates the arrangement of the following instruments and actions in a logical and meaningful order.
1. Lieutenant General
2. Brigadier
3. Colonel
4. Lieutenant
5. Field Marshal
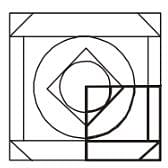
Which answer figure will complete the pattern in the question figure?

Which of the following is not an advantage of ICT tools?
ICT is a broad and comprehensive term. Information technologies are powered by mainly two types of communication technologies. Name them?
In order to help a mentally challenged child in your class, which of the following strategies would you adopt?
Mrs. Sen, science teacher of class 6, is the most liked teacher by the students of her class. This may be because
a. she answers each and every question asked by the children
b. she always practises child pedagogy while teaching
c. she is always decked in beautiful attires
d. she employs a lot of teaching aids in her teaching
In junior section, Mrs Singh is teaching about Earth, its shape and rotation, by using a globe. In such teaching, learning takes place by
Consider the following reaction 2A + 3F2 → 2AF3.
What is the formula for the reaction product if we substitute iodine for fluorine?
MnO4- + S2- + H+ → Mn2+ +S + H2O When this equation is balanced, the total number of coefficients on the left hand side are
When 20 g of naphthoic acid (C11H8O2) is dissolved in 50 g of benzene, a freezing point depression of 2 K is observed. [Kf (benzene) = 1.72Kmol-1 kg]. The van’t Hoff factor (i) is
[IIT - JEE 2007]
Ferric hydroxide is a negative sol, which of the following electrolyte will coagulate it most:
Enthalpies of formation of CO(g) , CO2 (g) , N2O (g) and N2O4 (g) are -110, - 393, 81 and 9.7 kJ mol-1. Thus, ΔrU for the reaction at 298 K is,
The rate of chemical reaction becomes double for every 10o rise in temperature because of
An element has configuration 4d55s2. The element belongs to
Among the following statements the one that is not true about Mendeleev’s Periodic Table is:
When a chemical bond is formed, there is decrease in
What’s the name of the 109th element as per the nomenclature?
Benzene and toluene form nearly ideal solutions. At 20ºC, the vapour pressure of benzene is 75 torr and that of toluene is 22 torr. The partial pressure of benzene at 20ºC for a solution containing 78 g of benzene and 46 g of toluene in torr is
[AIEEE-2005]
The actinoids Exhibit more member of oxidation states in general than the lanthanoids. This is because
"A young child responds to a new situation on the basis of the response made by him/ her in a similar situation as in the past". This is related to:
In the following question, out of the four alternatives, select the alternative which is the best substitute for the phrase.In the following question, out of the four alternatives, select the alternative which is the best substitute for the phrase.
Q. A man who is courteous and gallant in his behavior.