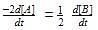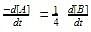NVS PGT Chemistry Mock Test - 9 - NVS TGT/PGT MCQ
30 Questions MCQ Test - NVS PGT Chemistry Mock Test - 9
Sandhya is sister of Praveena. How is Praveena’s father’s sister’s mother related to Sandhya?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
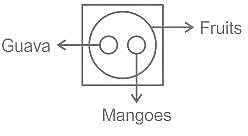
Select the Venn diagram that represents the given set of classes.
Guava, Mangoes, Fruits
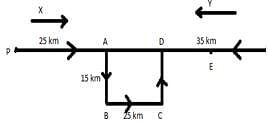
Two buses start from the opposite points of a main road, 150 km apart. The first bus runs for 25 km and takes a turn right and runs for 15 km. It then turns left and runs for another 25 km and takes the direction back to reach the main road. In the meantime, due to a minor breakdown, the other bus has run only 35 km along the main road, what would be the distance between the two buses at this point?
Select the number which can be placed at the sign of the question mark (?) from the given alternatives.
Mrs. Suman cannot score her students objectively. She is probably using
For a reaction 1/2 A→ 2B, rate of disappearance of ‘A’ related to the rate of appearance of ‘B’ by the expression -
[AIEEE 2008]
The maximum number of electrons that can have principal quantum number, n = 3 and spin quantum number,
Out of Cr (VI) as
, which is better oxidising agent?
2-Hexyne gives trans -2- Hexene on treatment with -
[AIEEE 2012]
In which of the following the stability of two oxidation states is correctly represented?
Among the following compounds the maximum number of lone pair is present on the central atom of:
Arrange the following in increasing order of boiling points.
I. 3 -methyl pentane
II. 3-chloropentane
III. 3-bromopentane
IV. 3,3-dichloropentane
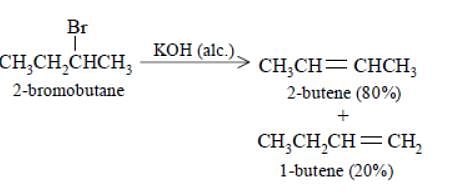
Elimination of bromine from 2–bromobutane results in the formation of –
[AIEEE-2005]
Matching List Type
Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d), out of which one is correct
Q.
Match the different solutions in Column I with their ΔTb in Column II and select the answer from the codes given below.
Direction (Q. Nos. 20-21) Choice the correct combination of elements and column I and coloumn II are given as option (a), (b), (c) and (d), out of which ONE option is correct.
Q.
Consider the molecules in Column I and match them with their stereochemical properties from Column II

C7H7Cl shows how many benzenoid aromatic isomers?
What is the order of a reaction which has a rate expression ; Rate =
Alkaline earth metals combine with halogens to form:
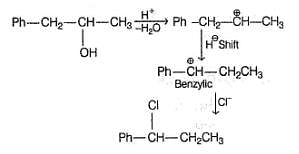
An organic compound X (C9H12O) gives the following reactions :
i. Na - Slow gas bubble formation
ii. Acetic anhydride - Pleasent smelling liquid
iii. CrO3-H2SO4 - Blue-green solution
iv. Hot KMnO4 - Benzoic acid
v. Br2-CCI4 - No decolouration
vi. I2 + NaOH - Yellow solid is formed
vii. X rotates the plane polarised light
Q.
If X is treated with HCI in the presence of ZnCI2, the major product would be
Water has a maximum density at _____ degree centigrade.
The conversion of primary aromatic amines into diazonium salts is known as:
Directions: In the following question, a sentence has been given in Direct/Indirect speech. Out of the four alternatives suggested, select the one which best expresses the same sentence in Indirect/Direct speech.
Q. The team leader said, “My team can win this tournament.”
In the following question, a sentence has been given in Active/Passive voice. Out of four alternatives suggested, select the one which best expresses the same sentence in Passive/Active voice.
Q. Why are they laughing at you?



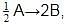 rate of disappearance of A is related to rate of appearance of B by the expression:
rate of disappearance of A is related to rate of appearance of B by the expression: