NVS PGT Chemistry Mock Test - 10 - NVS TGT/PGT MCQ
30 Questions MCQ Test - NVS PGT Chemistry Mock Test - 10
Hamzanama paintings were produced during the reign of?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Out of the given number pairs, three are similar in a certain manner. However, one pair is NOT like the other three. Select the pair which is different from the rest
In order to perform a calculation in a spreadsheet, you need to use a:
Which toolbar allows you to enter values and formulas?
What is the small triangle which draws lines on the logo screen called?
What is the file extension of a MS-Power Point file?
Naresh loves to work without supervision and set goals that are challenging, but not impossible. He learns best in solitude.
Which of the following sentences is true about Naresh?
After subsiding a disturbance in the classroom, the teacher`s main concern should be
Mr. Shyam is newly appointed as a teacher in school. Before delivering the lectures to the students, which of the following methods should Mr. Shyam adopt?
In the electrosynthesis,potassium manganate (VII) is converted to manganese(IV) dioxide. By passage of 1F of electrolysis ,one mole of potassium manganate(VII) will form manganese dioxide.
Comprehension Type
Direction (Q. Nos. 15-17) This section contains a paragraph, describing theory, experiments, data, etc. Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
Glycol when heated with concentrated H2SO4, undergo a variety of reactions as
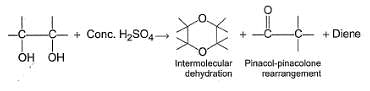
Nature of predominant product depends upon the nature of other groups present at the two α-carbons.
Q.
Which is most likely to undergo intermolecular dehydration to give dioxane or substituted dioxane?
The exothermic formation of ClF3 is represented by the equation :
Cl2(g) + 3F2(g) 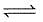 2ClF3(g) ; ΔH = -329 kJ
2ClF3(g) ; ΔH = -329 kJ
Which of the following will increase the quantity of ClF3 in an equilibrium mixture of Cl2, F2 and ClF3 :
A " 1/4 HP" electric motor uses 187 W of electrical energy while delivering 35 J of work each second. How much energy must be dissipated in the form of friction (heat)?
A 5.25% solution of a substance is isotonic with a 1.5% solution of urea (molar mass = 60 g mol-1) in the same solvent. If the densities of both the solutions are assumed to be equal to 1.0 gcm-3, molar mass of the substance will be-
[AIEEE 2007]
Mendeleev's Periodic Table was arranged primarily based on which property of elements?
In which of the following complexes, the nickel metal is in the highest oxidation state?
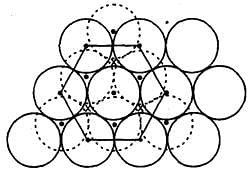
In hexagonal close packing of sphere in three dimensions.
What is the electronic configuration of carbon in it’s excited state?
What type of interaction hold the molecules together in a polar molecular solid?
In order to help a mentally challenged child in your class, which of the following strategies would you adopt?
Improve the bracketed part of the sentence with the parts given below.
Q. The government (to launch) a new scheme for women empowerment soon.
“भगवान श्रीराम के अयोध्या लौटने पर प्रजा ने खुशियाँ मनाई थी।” वाक्य में रेखाँकित शब्द के लिए सही मुहावरा है-


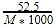 The molar concentration of urea solution is
The molar concentration of urea solution is 

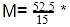 60 = 210.0 g/mol.
60 = 210.0 g/mol.