Bihar STET Paper 2 Chemistry Mock Test - 3 - Bihar PGT/TGT/PRT MCQ
30 Questions MCQ Test - Bihar STET Paper 2 Chemistry Mock Test - 3
The oxidation number of an element in a compound is evaluated on the basis of certain rules. Which of the following rules is not correct in this respect?
Passage I
A constant current of 30 A is passed through an aqueous solution of NaCl for a time of 1.00 h.
Thus Cl2 formed under STP condition is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the following elements has the largest ionisation enthalpy?
Yellow colour of NaCl crystals in sodium vapour is due to
A sub-shell with n = 6 , l = 2 can accommodate a maximum of
According to Bohr's theory, the angular momentum of an electron in 5th orbit is - [AIEEE 2006]
Consider the following reaction,
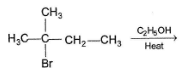
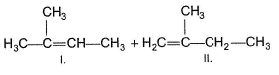
Q.
The correct statement concerning I and II is
Which of the following is the strongest Lewis base?
Uncertainty in position of a particle of 25 g in space is 10-5 m. Hence uncertainty in velocity (ms-1) is (Planck's constant h = 6.6 × 10-34 Js) [AIEEE- 2002]
The metal that can not be obtained by electrolysis of an aqueous solution of its salt is
[JEE Main 2014]
If equilibrium constant of
CH3COOH + H2O CH3COO- + H3O+
Is 1.8 × 10-5, equilibrium constant for
CH3COOH + OH- CH3COO- + H2O is
Correct IUPAC name of the following compound is :
Which among the following is an instantaneous reaction?
Glucose solution is one molal. Glucose present in 1 kg glucose solution is
Consider the following reaction 2A + 3F2 → 2AF3.
What is the formula for the reaction product if we substitute iodine for fluorine?
Benzene and toluene form nearly ideal solutions. At 20ºC, the vapour pressure of benzene is 75 torr and that of toluene is 22 torr. The partial pressure of benzene at 20ºC for a solution containing 78 g of benzene and 46 g of toluene in torr is
[AIEEE-2005]
According to Werner’s theory , the primary valences of the central atom
An element has configuration 4d55s2. The element belongs to
An aqueous solution is 6% methanol, CH3OH, by mass with d = 0.988 g mL-1. Thus, molarity of CH3OH in this solution is
'If you are given 100 rupees, what will you do?' The objective of asking this type of question is
A classroom having ______, clearly shows that the classroom studies are lively.
"A young child responds to a new situation on the basis of the response made by him/ her in a similar situation as in the past". This is related to:
Which of the following is a significant cause for the failure of a teacher?
Learners are asked to listen to a set of five sentences from their Environmental Studies textbook as the teacher reads out the sentences twice. Then the learners are asked to discuss in their groups to recall/recreate the sentence, not necessarily the exact sentence, but nearer to the sentences read out. What is this task known as?
In the QS World University Rankings 2024, what is IIT Bombay's global ranking in engineering?
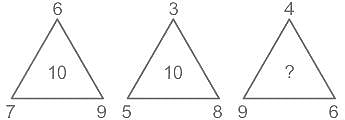
Select the number which can be placed at the sign of the question mark (?) from the given alternatives.