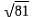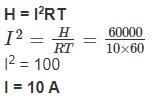Indian Army Agniveer Technical Mock Test - 1 - Indian Army Agniveer MCQ
30 Questions MCQ Test - Indian Army Agniveer Technical Mock Test - 1
Droupadi Murmu, who recently became the first woman tribal President of India, belongs to which tribe?
Sarojini Naidu was elected first Indian woman President of the Indian National Congress at ______.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
If 9 + 7 = 58 and 3 + 11 = 124, then 13 + 5 is equal to-
A box provides Rs. 77 in the denominations of one rupee, 50 paise, and 25 paise coins. The number of 50 paise coins is double that of 25 paise coins and four times that of one rupee coins. How many 50 paise coins are there in box?
Suppose A1 , A2 , ..., A30 are thirty sets each having 5 elements and B1, B2, ..., Bn are n sets each with 3 elements, let 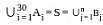 and each element of S belongs to exactly 10 of the Ai ’s and exactly 9 of the B's. then n is equal to
and each element of S belongs to exactly 10 of the Ai ’s and exactly 9 of the B's. then n is equal to
Consider the following statements:
I: If A = {x: x is an even natural number} and B = {y: y is a natural number}, A subset B.
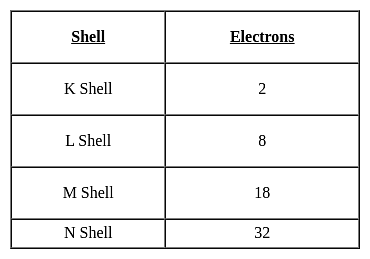
II: Number of subsets for the given set A = {5, 6, 7, 8) is 15.
III: Number of proper subsets for the given set A = {5, 6, 7, 8) is 15.
Which of the following statement(s) is/are correct?
If 2log(x+3) = log81 then the value of x.
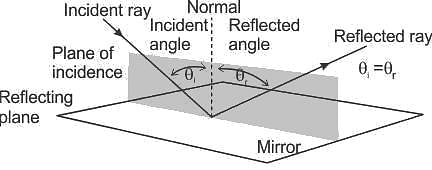
If the angle of incidence formed on a concave mirror at a point is 30° then the angle of reflection will be:
How much current is to be passed through a wire of resistance 10 Ω for a time of one minute to produce a heat of energy of 60,000 J ?
Which of the following is correct about reaction of phosphorus with water ?
Which one of the following methods is used to separate salt from seawater?


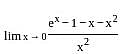 =
=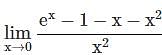
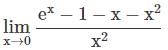
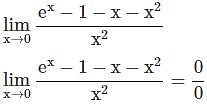
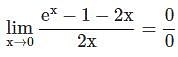
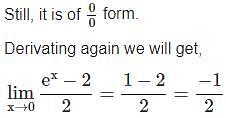
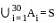
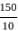 = 15
= 15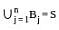
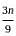 =
= 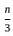
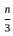 = 15
= 15