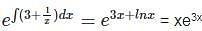Indian Army Agniveer Technical Mock Test - 5 - Indian Army Agniveer MCQ
30 Questions MCQ Test - Indian Army Agniveer Technical Mock Test - 5
Select the Venn diagram that best represents the relationship between the following classes.
Mammals, Zebra, Animals
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the following leaders opposed the Champaran satyagraha of Mahatma Gandhi ?
Words given on the left side of (::) are related with each other by some Logic/Rule /Relation. Select the missing word/word pair on the right side of (::) from the given alternatives based on the same Logic/Rule/Relation.
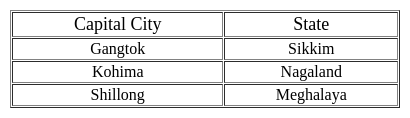
Ranchi : Jharkhand :: Dispur : ?
The integrating factor of the differential equation 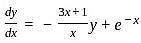 is
is
An electron and proton enter a magnetic field with equal velocities. Which one of them experiences a greater force?
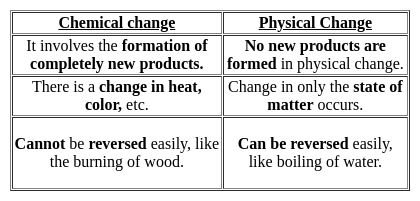
When carbon dioxide dissolves in water, it gives______?
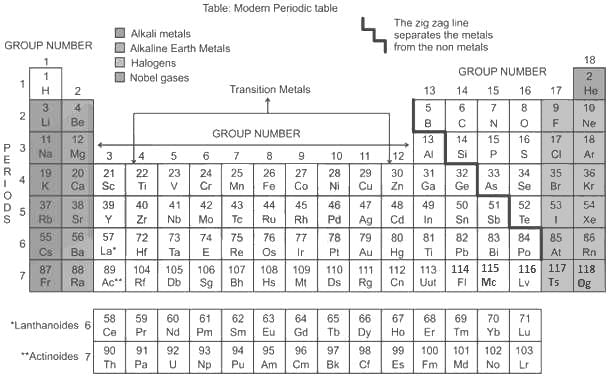
The atomic number of which of the following elements is more than that of phosphorus?



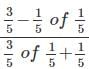 is equal to
is equal to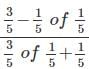
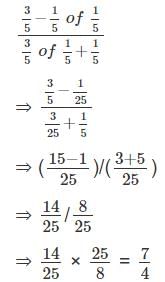
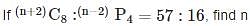
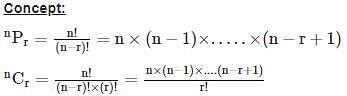
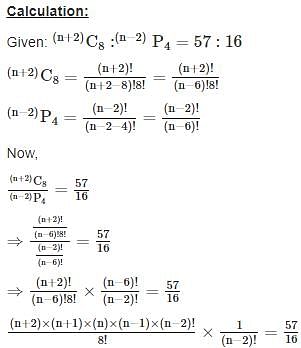
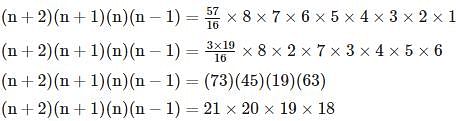
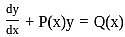 is given by
is given by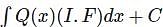
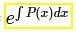
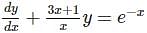
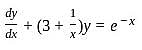 which is linear.
which is linear.