Indian Army Agniveer Technical Mock Test - 7 - Indian Army Agniveer MCQ
30 Questions MCQ Test - Indian Army Agniveer Technical Mock Test - 7
What is the currency of United Kingdom?
What is the full name of the Chinese army which is called PLA?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the following organisation has developed the Arjun MBT Mk IA battle tank?
Work can be finished by A, B, and C in 12, 24, and 36 days, respectively. How many days will they need to work together to finish the same task?
Evaluate the definite integration
∫04(x + 1) dx
and choose the right answer:
The area (in square units) of the triangle formed by the two ends of a latus rectum and a focus of the ellipse 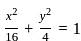 , is:
, is:
If A = 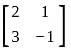 and I =
and I = 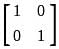 . Which of the following is the zero matrix?
. Which of the following is the zero matrix?
When the force is doubled and the area is halved, then the pressure will:
Heat energy produced by sun reaches to earth due to __________.
Which one of the following equations is the balanced chemical equation for the given reaction?
Fe + H2O → Fe3O4 + H2
Which of the following is not endothermic ?
The compound given below is called as a


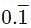 into rational fraction.
into rational fraction.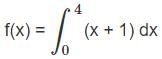
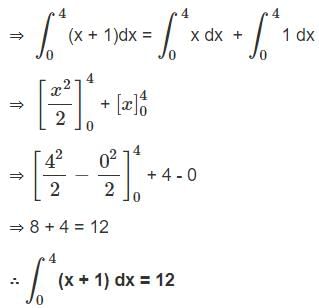
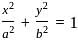 , where a = semi-major axis, and b = semi-minor axis.
, where a = semi-major axis, and b = semi-minor axis.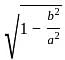
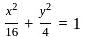
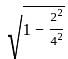
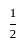 × AB × FF'
× AB × FF'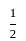 × 2 × 4√3
× 2 × 4√3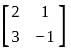 and I =
and I = 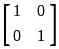 .
.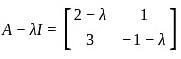
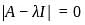
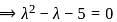
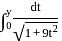 and
and 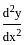 = ay, then a is equal to
= ay, then a is equal to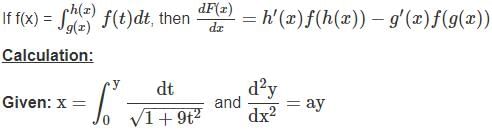
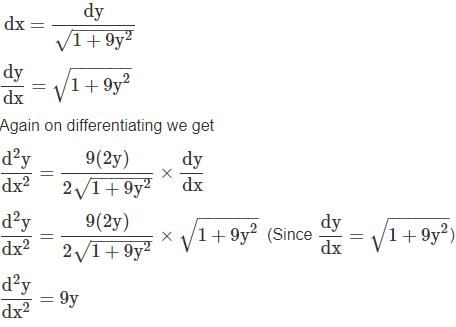
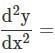 ayd2ydx2=ay we get
ayd2ydx2=ay we get
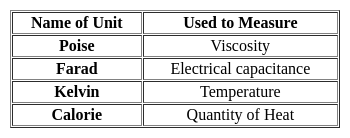
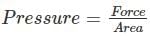
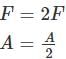
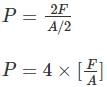
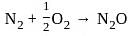 , when N2 reacts with O2, heat is absorbed as the sum of N-N and O=O bond energy is much greater than the N-O-N bond.
, when N2 reacts with O2, heat is absorbed as the sum of N-N and O=O bond energy is much greater than the N-O-N bond.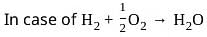
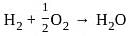 is not an endothermic process.
is not an endothermic process.














