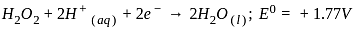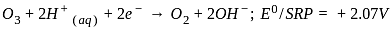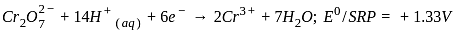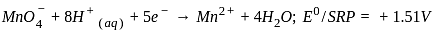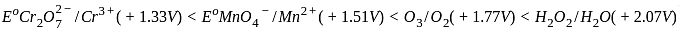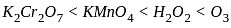MPTET Varg 2 Science Mock Test - 4 - MPTET MCQ
30 Questions MCQ Test - MPTET Varg 2 Science Mock Test - 4
<p>This is the pie chart of Teacher training program. In which total students 24.</p> <p><img alt="" src="https://edurev.gumlet.io//storage.googleapis.com/tb-img/production/22/09/F1_Mamata_SSC_16.09.22_ G1.PNG" style="width: 280px; height: 323px;" /></p> <p>What is the ratio of Quant students and all other students?</p>
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
“Learner learns from his own mistakes.” This statement is based on which learning theory?
How can a teacher promote gender stereotype flexibility in her classroom?
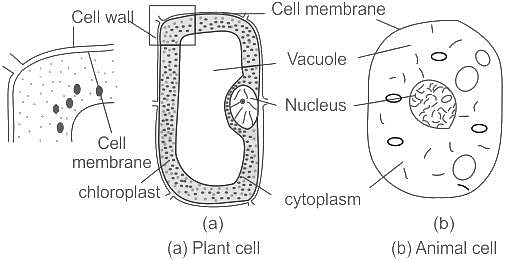
The location of nucleus in a typical plant cell is
Dalton’s atomic theory successfully explained
(i) Law of conservation of mass
(ii) Law of constant composition
(iii) Law of radioactivity
(iv) Law of multiple proportion
The potential difference is 10 V and the work done is 40 J. Find the electric charge that flow through the circuit.
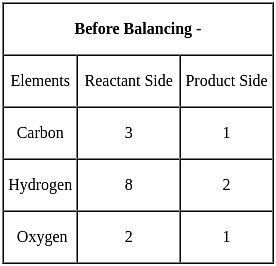
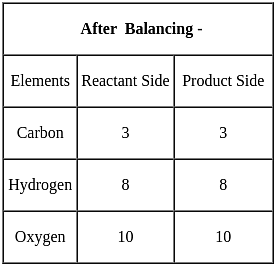
a C3H8(g) + b O2(g) → d CO2(g) + e H2O(l)
For the above balanced equation, a, b, d and e are respectively:
Which of the following should NOT be an objective of science education at the upper primary level?
Which of the following is NOT a characteristic of a Competency-Based Approach?
A) The students are practicing the multiplication table chart.
B) Individual students are learning at their own pace.
C) They are taking peer assistance in the group.
How many moles of CO can be obtained by reacting 2.0 mole of CH4, with 2.0 mole of O2, according to the equation given below?
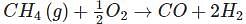
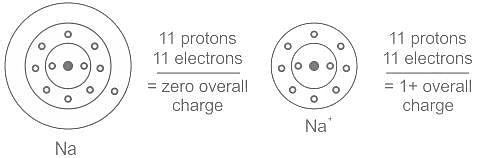
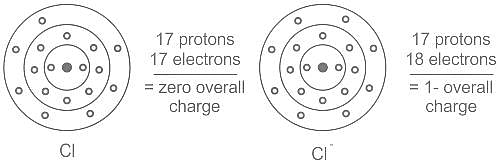
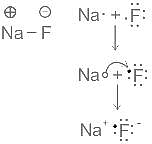
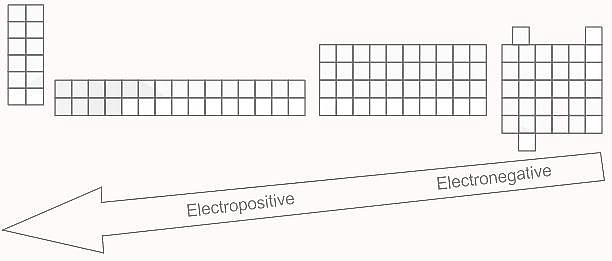
Element X is strongly electropositive and element Y is strongly electronegatvie. Both are univalent. The compound formed would be:
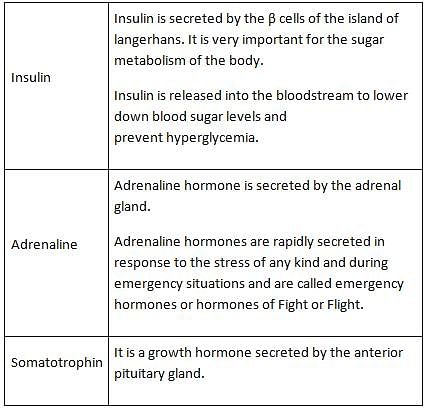
'Diabetes insipidus' is due to the deficiency of hormone
Which one of the features mentioned below about decomposers is not applicable to decomposers?
Which of the following statement is incorrect?
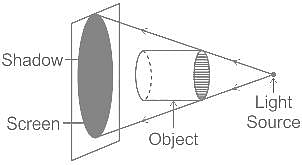
Which of the following properties of light can be used to explain the phenomenon of shadow formation?


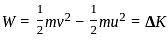
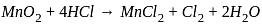 , the oxidizing agent is MnO
, the oxidizing agent is MnO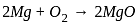 , the oxidizing agent is O
, the oxidizing agent is O