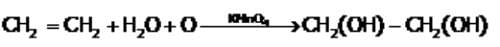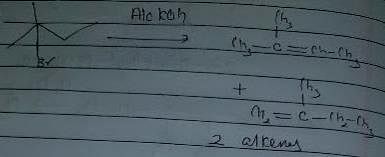Test: Alkanes & Alkenes(9- Sep) - JEE MCQ
10 Questions MCQ Test - Test: Alkanes & Alkenes(9- Sep)
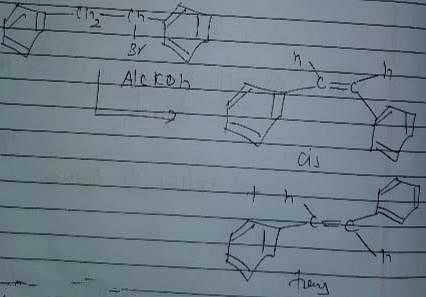
Statement I : 2, 3-dibromo butane with Zn-dust gives frans-2-butene as major product.
Statement II : frans-2-butene is more stable than c/s-2-butene.
Statement I : 2-bromobutane with (CH3)3COK in tertiary butanol gives 1 -butene as major product.
Statement II : Very strong base (CH3)3COK in tertiary butanol brings about E-2 elimination reaction.
Statement II : Very strong base (CH3)3COK in tertiary butanol brings about E-2 elimination reaction.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
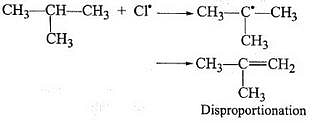
During free radical bromination of isobutane, an alkene is produced as by product via disproportionation of the intermediate alkyl free radical. What is this alkene?
Alkenes react with water in presence of a few drops of conc. sulphuric acid to form:
Which is the best description of Hammond postulate?
What is the major bromination product in the following reaction?
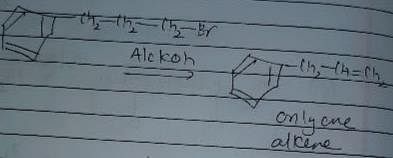
In which of the following reactions , only single is omer of alkene is formed ?
Statement I : Boiling point of an alkene is slightly greater than that of its hydrogenated alkane.
Statement II : Alkenes have stronger van der Waals’ force of attraction than alkanes.