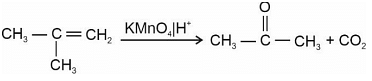Test: Hydrocarbons(12 Sep) - JEE MCQ
15 Questions MCQ Test - Test: Hydrocarbons(12 Sep)
Given below are two statements.
Statement I: The presence of weaker  -bonds make alkenes less stable than alkanes.
-bonds make alkenes less stable than alkanes.
Statement II: The strength of the double bond is greater than that of carbon-carbon single bond.
In the light of the above statements, choose the correct answer from the options given below.
Statement I: The presence of weaker
Statement II: The strength of the double bond is greater than that of carbon-carbon single bond.
In the light of the above statements, choose the correct answer from the options given below.
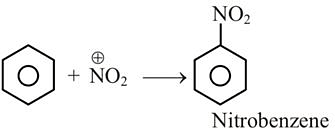
Benzene on nitration gives nitrobenzene in presence of HNO3 and H2SO4 mixture, where
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Complete combustion of 3 g of ethane gives x × 1022 molecules of water. The value of x is ______. (Round off to the nearest integer)
[Use: NA = 6.023 × 1023; Atomic masses in u: C: 12.0; O: 16.0; H: 1.0]
Methylation of 10 g of benzene gave 9.2 g of toluene. Calculate the percentage yield of toluene _________. (Nearest integer)
An unsaturated hydrocarbon X absorbs two hydrogen molecules on catalytic hydrogenation, and also gives the following reaction:

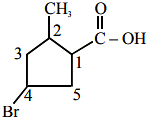
B(3-oxo-hexanedicarboxylic acid) X will be:
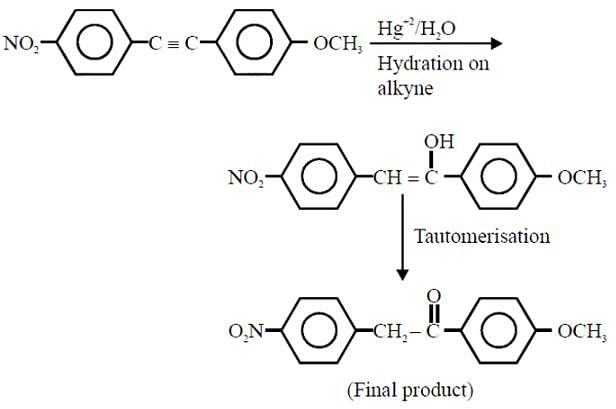
The major product obtained from the following reaction is
In the following sequence of reactions, the maximum number of atoms present in molecule 'C' in one plane is __________. (In integer)
(A is the lowest molecular weight alkyne.)
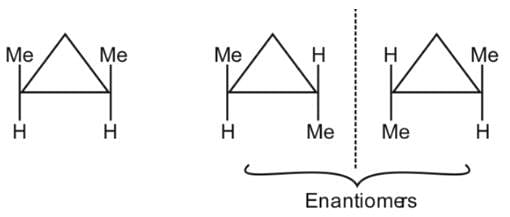
The number of stereoisomers possible for 1,2-dimethyl cyclopropane is:
For the following sequence of reactions, the correct products are:
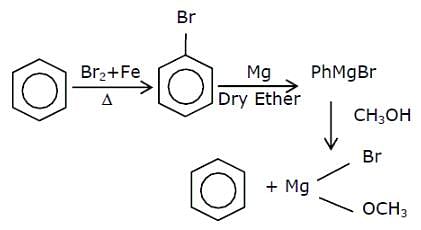
In the chemical reaction given below, A and B respectively are:
Two isomers 'A' and 'B' with molecular formula C4H8 give different products on oxidation with KMnO4 in acidic medium. Isomer 'A' on reaction with KMnO4/H+ results in effervescence of a gas and gives ketone. The compound 'A' is:
Arrange the following conformational isomers of n-butane in order of their increasing potential energy:


 -bond present in alkenes is weaker than
-bond present in alkenes is weaker than  -bond present in alkanes. This makes alkenes less stable than alkanes. Therefore, Statement I is correct.
-bond present in alkanes. This makes alkenes less stable than alkanes. Therefore, Statement I is correct.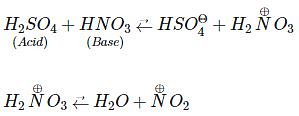
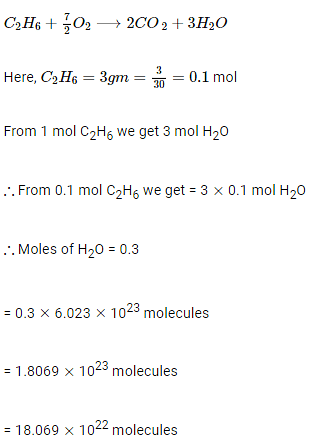
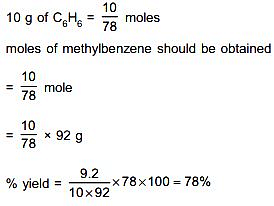


 electron rule, it is non-aromatic due to its nonplanar nature. It is nonplanar due to repulsion of C-H bonds present inside the ring.
electron rule, it is non-aromatic due to its nonplanar nature. It is nonplanar due to repulsion of C-H bonds present inside the ring.