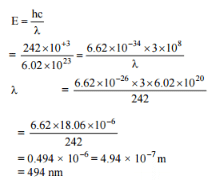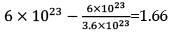AWES TGT Chemistry Mock Test - 9 - AWES TGT/PGT MCQ
30 Questions MCQ Test - AWES TGT Chemistry Mock Test - 9
In which state was the Swaminarayan Institute of Medical Science and Research inaugurated by Amit Shah?
Which state government approved 10% reservation for Paharis and added 15 new castes to the Other Backward Classes (OBCs)?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which bank secured seven awards at the 19th Banking Tech Conference?
What is the name of the world's largest iceberg that has recently begun an unusual journey?
According to the Organisation for Economic Co-operation and Development (OECD), which country emerges as the foremost aid provider in 2022?
One’s ability to differentiate between right and wrong is known as:
Johnson is an experienced teacher. He came to the class yesterday and told his students that they have reached the stage at which they are capable of thinking rationally about events and objects. Which stage is Johnson referring to at which a child starts thinking logically about events and objects?
The inner force that stimulates and compels a behavioral response and provides specific direction to that response is:
How should a newly appointed teacher deal with the situation, if his students don't obey him?
What happens to the ions, when an ionic compound dissolves in a solvent?
A " 1/4 HP" electric motor uses 187 W of electrical energy while delivering 35 J of work each second. How much energy must be dissipated in the form of friction (heat)?
At what does the following cell have its reaction at equilibrium?
Ag(s) | Ag2CO3(s) | Na2CO3 (aq) || KBr(aq) | AgBr(s) | Ag(s)
KSP = 8 x 10 – 12 for Ag2CO3 and KSP = 4 x 10 – 13 for AgBr
What is the electronic configuration of carbon in it’s excited state?
The maximum number of atomic orbitals associated with a principal quantum number 5 is
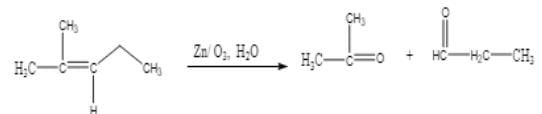
Ozonolysis of an organic compound ‘A’ produces acetone and propionaldehyde in equimolar mixture. Identify ‘A’ from the following compounds :
[AIEEE 2011]
The energy required to break one mole of Cl - Cl bonds in Cl2 is 242 kJ mol-1. The longest wavelength of light capable of breaking a single Cl - Cl bond is(c = 3 x 108 ms-1 and NA = 6.02 x 1023 mol-1) [AIEEE 2010]
The factors which are responsible for the stability of lyophilic sols are:
Which among the following is not the method of colloidal purification?
Reaction
+ HOCl → product,
here product will be -
[AIEEE-2002]
Only One Option Correct Type
Direction (Q. Nos. 6-15) This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
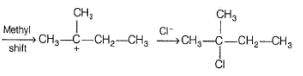
Which is the major product in the following reaction?
Mendeleev's Periodic Table was arranged primarily based on which property of elements?
How much time (in hours) would it take to distribute one Avogadro number of wheat grains if 1020 grains are distributed each second?
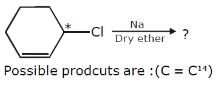
What is the product formed in the following reaction?