Test: MOT and its properties - JEE MCQ
15 Questions MCQ Test - Test: MOT and its properties
On changing 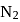 to
to 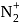 , the dissociation energy of
, the dissociation energy of 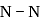 bond
bond 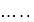 and on changing
and on changing 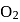 to
to 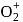 the dissociation energy of O-O bond....
the dissociation energy of O-O bond....
Which of the following is correct increasing order of lone pair of electrons on the central atom?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
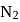 and
and 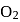 are converted into monocations,
are converted into monocations, 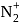 and
and 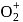 respectively. Which of the following statements is wrong?
respectively. Which of the following statements is wrong?
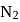 and
and 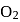 are converted into monocations,
are converted into monocations, 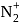 and
and 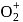 respectively. Which of the following statements is wrong?
respectively. Which of the following statements is wrong?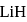 is
is 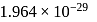 Coulomb meter and the interatomic distance between
Coulomb meter and the interatomic distance between 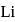 and
and 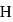 is 1.596 Å. The percentage ionic character of
is 1.596 Å. The percentage ionic character of 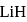 is
is
Which one of the following formulae does not correctly represent the bonding capacities of the two atoms involved?
Arrange the following ions in the order of decreasing X−O bond length, where X is the central atom
Which among the following are having diamagnetic property?
(i) 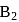
(ii) 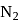
(iii) 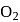
(iv) 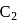
The electronic configuration of four elements 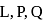 and
and 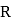 are given in brackets
are given in brackets
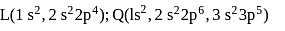
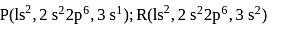
The formulae of ionic compounds that can be formed between these elements are
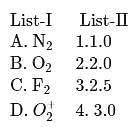
Match List I (species) and List II (bond orders) and select the correct answer:
The bond dissociation energy 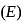 and bond length
and bond length 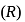 of
of 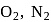 and
and 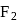 follow the order as:
follow the order as:
Which of the following species is isoelectronic with 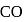 ?
?
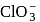 ion according to Valence Shell Electron Pair Repulsion (VSEPR) theory will be:
ion according to Valence Shell Electron Pair Repulsion (VSEPR) theory will be:


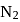 to
to 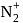 , B.O. decreases from 3 to
, B.O. decreases from 3 to 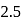 whereas on changing
whereas on changing 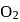 to
to 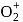 , B.O. increases from 2 to 2.5. In former case, the bond dissociation energy decreases and in the latter case, it increases.
, B.O. increases from 2 to 2.5. In former case, the bond dissociation energy decreases and in the latter case, it increases.
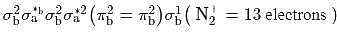
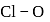 bond order in perchlorate ion is ___
bond order in perchlorate ion is ___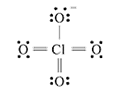
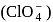 have the following possible resonating structures:
have the following possible resonating structures: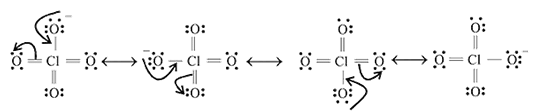
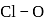 bond order
bond order 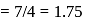
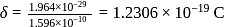
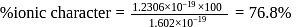
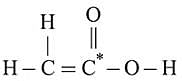
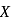 , lesser will be the bond length of
, lesser will be the bond length of 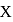 -O bond.
-O bond.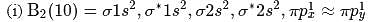
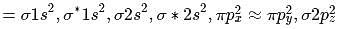
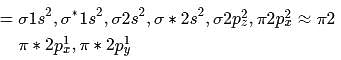
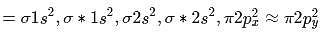
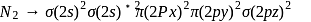 Bond order
Bond order 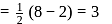
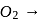 bond order
bond order 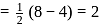
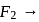 bond order
bond order 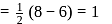
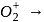 bond order
bond order 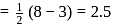
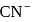 is
is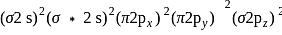
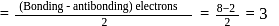
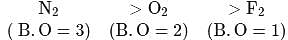
 has 3 bond pairs and 1 lone pair in
has 3 bond pairs and 1 lone pair in 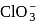 . So, it is pyramidal.
. So, it is pyramidal.














