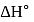Test: Thermochemistry, Entropy Changes - JEE MCQ
10 Questions MCQ Test - Test: Thermochemistry, Entropy Changes
If a reaction involves only solids and liquids which of the following is true?
One mole of a non-ideal gas undergoes a change of state (2.0 atm,3.0 L,95 K) → (4.0 atm, 5.0 L, 245 K) with a change in internal energy, ΔU = 30.0 L atm. The change in enthalpy ΔH of the process in L atm is.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
If bond enthalpies of 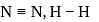 and
and 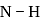 bonds are
bonds are 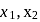 and
and 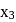 respectively,
respectively, 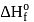 for
for 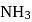 will be
will be
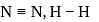 and
and 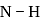 bonds are
bonds are 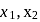 and
and 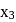 respectively,
respectively, 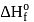 for
for 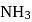 will be
will beHeat of neutralization of a strong acid HA and a weaker acid  with
with 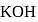 are
are 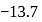 and
and 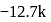 cal
cal 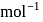 . When 1 mole of
. When 1 mole of 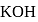 was added to a mixture containing 1 mole each of HA and
was added to a mixture containing 1 mole each of HA and  , the heat change was
, the heat change was 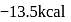 . In what ratio is the base distributed between HA and HB.
. In what ratio is the base distributed between HA and HB.
The latent heat of vapourization of a liquid at 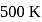 and 1 atm pressure is
and 1 atm pressure is  . What will be the change in internal energy
. What will be the change in internal energy 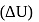 of 3 moles of liquid at the same temperature
of 3 moles of liquid at the same temperature
The difference between the reaction enthalpy change 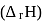 and reaction internal energy change
and reaction internal energy change 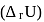 for the reaction:
for the reaction:
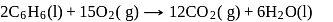
at 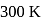 is
is 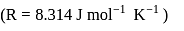
From given following equations and 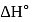 values, determine the enthalpy of reaction at
values, determine the enthalpy of reaction at 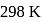 for the reaction :
for the reaction :
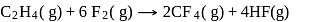
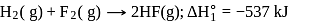
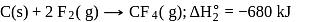
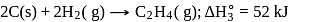
The enthalpy of the reaction forming 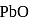 according to the following equation is
according to the following equation is 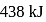 . What heat energy
. What heat energy  is released in formation of
is released in formation of 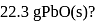
(Atomic masses : Pb = 207,O = 16.0)
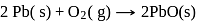
What is 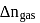 for the combustion of 1 mole of benzene, when both the reactants and the products are at
for the combustion of 1 mole of benzene, when both the reactants and the products are at 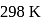 ?
?
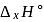 equal to
equal to 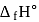 ?
?


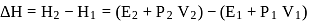
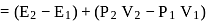
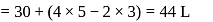 atm
atm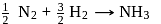
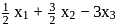
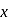 mole of
mole of 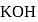 be neutralized by the strong acid
be neutralized by the strong acid 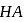 . Then, moles neutralized by
. Then, moles neutralized by 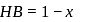
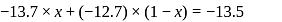
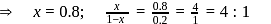
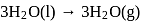
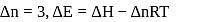
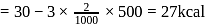
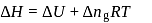
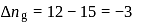
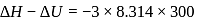
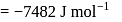
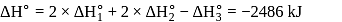
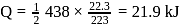
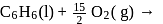
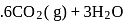 (l)
(l)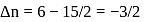
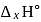 or
or