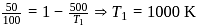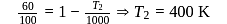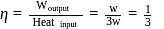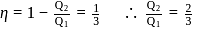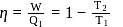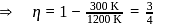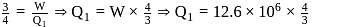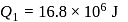Test: Carnot engine - JEE MCQ
10 Questions MCQ Test - Test: Carnot engine
If an air conditioner is put in the middle of a room and started working
In the following P−V diagram two adiabatics cut two isothermals at temperatures 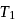 and
and 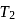 (fig). The value of
(fig). The value of 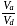 will be
will be
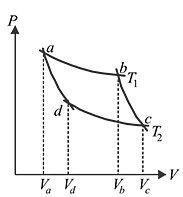
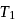 and
and 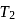 (fig). The value of
(fig). The value of 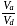 will be
will be
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
A heat engine absorbs heat 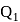 at temperature
at temperature 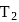 and heat
and heat 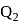 at temperature
at temperature 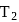 . Work done by the engine is
. Work done by the engine is 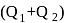 . This data:
. This data:
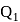 at temperature
at temperature 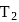 and heat
and heat 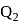 at temperature
at temperature 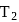 . Work done by the engine is
. Work done by the engine is 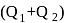 . This data:
. This data:A Carnot engine operating between temperatures 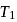 and
and 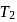 has efficiency
has efficiency 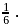 When
When 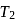 is lowered by
is lowered by 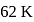 its efficiency increases to
its efficiency increases to 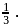 Then
Then 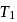 and
and 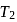 are, respectively
are, respectively
If the co-efficient of performance of a refrigerator is 5 and operates at the room temperature 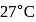 , the temperature inside the refrigerator is
, the temperature inside the refrigerator is
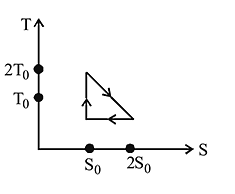
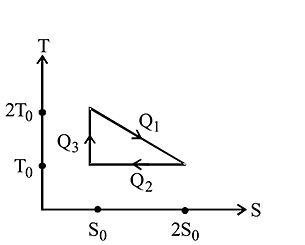
The temperature-entropy diagram of a reversible engine cycle is given in the figure. Its efficiency is
A Carnot engine whose efficiency is 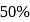 has an exhaust temperature of
has an exhaust temperature of 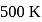 . If the efficiency is to be
. If the efficiency is to be 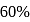 with the same intake temperature, the exhaust temperature must be (in K)
with the same intake temperature, the exhaust temperature must be (in K)
If the energy input to a Carnot engine is thrice the work it performs then, the fraction of energy rejected to the sink is
In a Carnot engine, the temperature of reservoir is 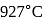 and that of
and that of 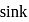 is
is 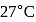 . If the work done by the engine when it transfers heat from reservoir to sink is
. If the work done by the engine when it transfers heat from reservoir to sink is 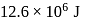 , the quantity of heat absorbed by the engine from the reservoir is
, the quantity of heat absorbed by the engine from the reservoir is


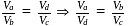
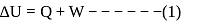
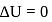 , for heat engines, because initial and final states are same. Internal energy is a state function.
, for heat engines, because initial and final states are same. Internal energy is a state function.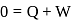
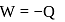
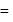
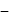 Total heat exchanged
Total heat exchanged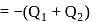
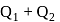
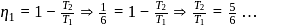
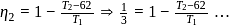
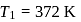 and
and 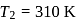
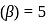
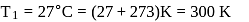
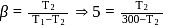
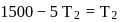 or
or 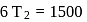
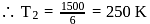
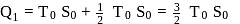
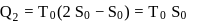 and
and 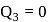
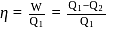
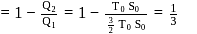
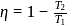 or
or