Test: Acid-Base Equilibrium - JEE MCQ
10 Questions MCQ Test - Test: Acid-Base Equilibrium
Statement A : pH of buffer increases with increasing temperature.
Statement B : The value of KW of water decreases with decreasing temperature.
Statement B : The value of KW of water decreases with decreasing temperature.
The number of species of the following that can act both as Bronsted acids and bases is  ,
, 
 ,
, 
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
 of
of  is added to
is added to 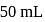 of
of  barium hydroxide solution. What is the
barium hydroxide solution. What is the  of the resulting solution?
of the resulting solution?
 of
of  is added to
is added to 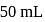 of
of  barium hydroxide solution. What is the
barium hydroxide solution. What is the  of the resulting solution?
of the resulting solution?Which equilibrium can be described as an acidbase reaction using the Lewis acid-base definition but not using the Bronsted-Lowry definition?
What is the decreasing order of basic strengths of 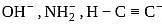 and
and 
Values of dissociation constant, are given as follows:

Correct order of increasing base strength of the base and will be:
Given
(i) 

(ii) 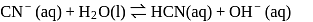

These equilibria show the following order of the relative base strength,
The degree of dissociation of  weak acid HA is
weak acid HA is 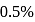 . If
. If  of
of 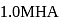 solution is diluted to
solution is diluted to  the degree of dissociation of acid and
the degree of dissociation of acid and  ion concentration in the resulting solution will be respectively
ion concentration in the resulting solution will be respectively
 can be explained on the basis of which of the following concepts?
can be explained on the basis of which of the following concepts?
The hydrogen ion concentration of 
 which is
which is  dissociated is
dissociated is


 also increases.
also increases. (Bronsted acid)
(Bronsted acid) (Bronsted base)
(Bronsted base)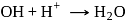 (Bronsted base)
(Bronsted base) (Bronsted acid)
(Bronsted acid)
 (Bronsted base)
(Bronsted base) (Bronsted acid)
(Bronsted acid)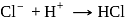 (Bronsted base)
(Bronsted base) and
and  (two species act as acid as well as base).
(two species act as acid as well as base).

 involves loss and gain of electrons.
involves loss and gain of electrons.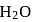 is coordinated to
is coordinated to  by donating electrons (LHS). It is then removed by withdrawing electrons.
by donating electrons (LHS). It is then removed by withdrawing electrons. conjugate base will follow the order
conjugate base will follow the order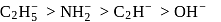
 lower will be the value of
lower will be the value of  i.e. higher will be the acidic nature. Further
i.e. higher will be the acidic nature. Further  and
and  are conjugate base of the acids
are conjugate base of the acids  and
and  respectively hence the correct order of base strength will be
respectively hence the correct order of base strength will be






 is
is 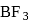 is electron deficient i.e, lone-pair of electrons acceptor (Lewis acid), because in
is electron deficient i.e, lone-pair of electrons acceptor (Lewis acid), because in  ,
,  is
is  hybridised and it does not satisfy octet (
hybridised and it does not satisfy octet ( 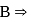 has say bonded electrons).
has say bonded electrons).

















