Test: Solubility - JEE MCQ
10 Questions MCQ Test - Test: Solubility
The solubility of 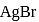 with solubility product
with solubility product 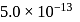 at
at  in
in  solution would be
solution would be
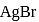 with solubility product
with solubility product 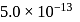 at
at  in
in  solution would be
solution would be| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
If the concentration of  ions in the saturated solution of
ions in the saturated solution of  is
is 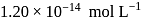 , then find the solubility product of
, then find the solubility product of 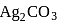 .
.
 ions in the saturated solution of
ions in the saturated solution of  is
is 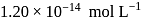 , then find the solubility product of
, then find the solubility product of 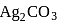 .
.If the molar solubility (in 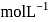 ) of a sparingly soluble salt
) of a sparingly soluble salt  is
is  and the corresponding solubility product is
and the corresponding solubility product is  , then
, then  in terms of
in terms of 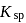 is given by the relation
is given by the relation
The solubility product of  is
is  .
.
What is the concentration of  ions in that solution (in gL−1)
ions in that solution (in gL−1)
What is the minimum concentration of  required to precipitate
required to precipitate  in a solution containing
in a solution containing 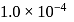 mole of
mole of  ?
?  for
for 
Calculate the molar solubility of calcium hydroxide Ca(OH)2 in 0.10MNaOH solution. The ionic product of calcium hydroxide is 5.5 × 10−6.
How many gms of  will dissolve in one litre of saturated solution.
will dissolve in one litre of saturated solution.  of
of  is
is 
Zirconium phosphate 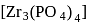 dissociates into three zirconium cations of charge
dissociates into three zirconium cations of charge  and four phosphate anions of charge
and four phosphate anions of charge 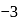 . If molar solubility of zirconium phosphate is denoted by S and its solubility product by
. If molar solubility of zirconium phosphate is denoted by S and its solubility product by  then which of the following relationship between
then which of the following relationship between  and
and  is correct?
is correct?
On addition of increasing amount of  to
to  each of
each of  and
and  in a solution, what
in a solution, what  of
of 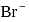 ion get precipitated when
ion get precipitated when  ion starts precipitating. K12sp(AgCl) = 1.0 × 10−10,,
ion starts precipitating. K12sp(AgCl) = 1.0 × 10−10,,



 is '
is ' 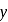 ' M. Its solubility product is
' M. Its solubility product is 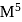 .
.



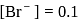 (from
(from 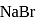 )
)





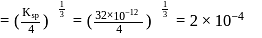


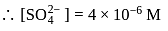

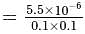

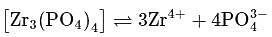



 required
required
 left at this stage
left at this stage 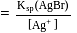

 of remaining
of remaining
 of
of  to be precipitated
to be precipitated 















