Test: Alkaline Earth Metals and its properties - JEE MCQ
20 Questions MCQ Test - Test: Alkaline Earth Metals and its properties
Aqueous solution of a group 2 element is precipitated by adding 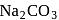 , then this precipitate is tested on flame, no light in visible region is observed, this element can be
, then this precipitate is tested on flame, no light in visible region is observed, this element can be
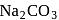 , then this precipitate is tested on flame, no light in visible region is observed, this element can be
, then this precipitate is tested on flame, no light in visible region is observed, this element can beAlkaline earth metals combine with halogens to form:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
 is soluble in water whereas fluorides of other alkaline earth metals are insoluble because of
is soluble in water whereas fluorides of other alkaline earth metals are insoluble because of
 is soluble in water whereas fluorides of other alkaline earth metals are insoluble because of
is soluble in water whereas fluorides of other alkaline earth metals are insoluble because ofWhich category of salts of alkaline earth metals is not found in solid sate, but found in solution state?
Among the given statements, the incorrect one is
Amongst the following hydroxides, the one which has the lowest value of  is :
is :
 reacts with water forming propyne gas.
reacts with water forming propyne gas.  ions has:
ions has:
A compound (A) is used in preparation of washing soda to recover ammonia in Solvay's process. When  is bubbled through an aqueous solution of
is bubbled through an aqueous solution of  , the solution turns milky. It is used in white washing due to disinfectant nature. What is the chemical formula of A?
, the solution turns milky. It is used in white washing due to disinfectant nature. What is the chemical formula of A?
Substance which absorbs 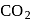 and violently reacts with
and violently reacts with 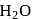 with Hissing sound is :
with Hissing sound is :
The metal  is prepared by the electrolysis of fused chloride. It reacts with hydrogen to form a colourless solid from which hydrogen is released on treatment with water. The metal is :
is prepared by the electrolysis of fused chloride. It reacts with hydrogen to form a colourless solid from which hydrogen is released on treatment with water. The metal is :
Two metals  and
and  belong to the same group of the periodic table. Metal (A) forms an insoluble oxide but a soluble sulphate, metal (B) forms a soluble oxide but an insoluble sulphate. Both metals (A) and (B) form hydroxides which are soluble in alkalis. (A) and (B) are
belong to the same group of the periodic table. Metal (A) forms an insoluble oxide but a soluble sulphate, metal (B) forms a soluble oxide but an insoluble sulphate. Both metals (A) and (B) form hydroxides which are soluble in alkalis. (A) and (B) are
Calcium imide on hydrolysis gives gas (B) which on oxidation by bleaching powder gives gas 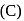 . Gas (C) on reaction with magnesium give compound (D) which on hydrolysis gives again gas (B). Identify (B), (C) and (D).
. Gas (C) on reaction with magnesium give compound (D) which on hydrolysis gives again gas (B). Identify (B), (C) and (D).
Which one is the correct statement with reference to solubility of  in water?
in water?
A solid compound '  ' on heating gives
' on heating gives  gas and a residue. The residue mixed with water forms 'Y'. On passing an excess of
gas and a residue. The residue mixed with water forms 'Y'. On passing an excess of  through '
through '  ' in water, a clear solution 'Z' , is obtained. On boiling 'Z', compound 'X' is reformed. The compound
' in water, a clear solution 'Z' , is obtained. On boiling 'Z', compound 'X' is reformed. The compound  is
is
The difference of number of water molecules in gypsum and plaster of Paris is
Which of the following alkaline earth metal sulphates is the most soluble in water?
What are the products formed when an aqueous solution of magnesium bicarbonate is boiled?
The thermal stability of  and
and 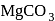 decreases in the order:
decreases in the order:


 due to its small size are tightly bound so they cannot be excited by the flame.
due to its small size are tightly bound so they cannot be excited by the flame. being small in size is heavily hydrated and heat of hydration exceeds the lattice energy. Hence
being small in size is heavily hydrated and heat of hydration exceeds the lattice energy. Hence  is soluble in water.
is soluble in water. ion exists as
ion exists as 
 is insoluble in water and thus has lower value of
is insoluble in water and thus has lower value of 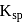
 ion is
ion is 
 bonds.
bonds. Cation Anion Anode
Cation Anion Anode 

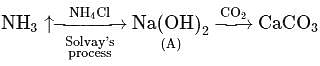

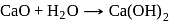
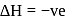


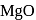 is insoluble, whereas
is insoluble, whereas 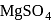 is soluble, whereas
is soluble, whereas 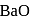 is soluble, but
is soluble, but  is insoluble.
is insoluble.
 is the only alkaline earth metal sulphate which is soluble in water and for solubility hydration energy should be greater than lattice energy.
is the only alkaline earth metal sulphate which is soluble in water and for solubility hydration energy should be greater than lattice energy.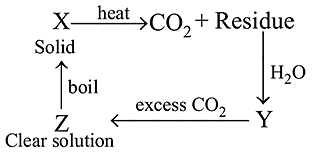


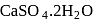 , where as plaster of paris is
, where as plaster of paris is 
 or
or 

 is soluble in water because
is soluble in water because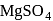 is soluble because its hydration energy is higher than the lattice energy.
is soluble because its hydration energy is higher than the lattice energy.














