Test: Nernst Equation, Conductance and Conductivity - JEE MCQ
15 Questions MCQ Test - Test: Nernst Equation, Conductance and Conductivity
Cell reaction is spontaneous when
Three cell A, B and C has equilibrium constant in the ratio 1:4 : 9 respectively. Arrange the following cells in the order of increasing Gibbs free energy.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Gibbs free energy change for a cell reaction is positive what does it indicates?
The emf of a particular voltaic cell with the cell reaction  is
is  V. The maximum electrical work of this cell when
V. The maximum electrical work of this cell when 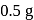 of
of 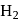 is consumed.
is consumed.
 are respectively :
are respectively :
The Nernst equation E = E∘−RT/nF, ln Q indicates that the Q will be equal to equilibrium constant Kc when :
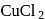 solution, the mass of the cathode increased by
solution, the mass of the cathode increased by  . What occured at the copper anode?
. What occured at the copper anode?
 of metal was deposited when a current of 2 ampere is passed through a metal ion solution for 100 seconds. What is the electrochemical equivalent (in gram coulomb -1) of the metal?
of metal was deposited when a current of 2 ampere is passed through a metal ion solution for 100 seconds. What is the electrochemical equivalent (in gram coulomb -1) of the metal?
Electrode potentials (E∘) are given below :

Based on the above potentials, strongest oxidizing agent will be:
 is reduced by electrolysis at low potentials and high currents. If
is reduced by electrolysis at low potentials and high currents. If  amperes of current is passed through molten
amperes of current is passed through molten  for 6 hours, what mass of aluminium is produced? (Assume
for 6 hours, what mass of aluminium is produced? (Assume  current efficiency. At. mass of
current efficiency. At. mass of  )
)
Assertion (A) : A current of  is passed into aqueous
is passed into aqueous 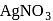 solution for
solution for  . The weight of silver deposited is
. The weight of silver deposited is  (At. wt. of
(At. wt. of 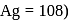
Reason (R): The mass of a substance deposited during the electrolysis of an electrolyte is inversely proportional to the quantity of electricity passing through the electrolyte.
The correct answer is :
 Would be Given
Would be Given 
 , and
, and 
 is electrolysed using platinum electrodes. The reaction occurring at anode is
is electrolysed using platinum electrodes. The reaction occurring at anode is










 or
or 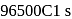 the charge carried by
the charge carried by 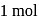 of electrons when water is electrolysed
of electrons when water is electrolysed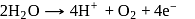
 of
of  Thus 1 Faraday of electricity liberate
Thus 1 Faraday of electricity liberate of
of  of
of 
 ions is
ions is 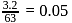 moles.
moles. mole of
mole of  must pass into solution from anode by oxidation
must pass into solution from anode by oxidation
 coulomb
coulomb will be strongest oxidizing agent.
will be strongest oxidizing agent.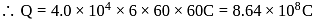
 of
of 

 of
of 




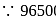 coulomb deposited wt. of
coulomb deposited wt. of 
 coulomb deposited wt. of
coulomb deposited wt. of 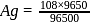
 is true but
is true but  is false.
is false.  is false because mass of substance deposited is directly proportional to the quantity of electricity.
is false because mass of substance deposited is directly proportional to the quantity of electricity.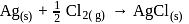

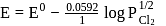


 Useful work
Useful work 


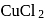 , At anode,
, At anode,















