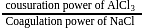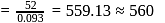Test: Emulsions and Gels. - JEE MCQ
Test Description
15 Questions MCQ Test - Test: Emulsions and Gels.
Test: Emulsions and Gels. for JEE 2024 is part of JEE preparation. The Test: Emulsions and Gels. questions and answers have been prepared
according to the JEE exam syllabus.The Test: Emulsions and Gels. MCQs are made for JEE 2024 Exam.
Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Emulsions and Gels. below.
Solutions of Test: Emulsions and Gels. questions in English are available as part of our course for JEE & Test: Emulsions and Gels. solutions in
Hindi for JEE course.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free. Attempt Test: Emulsions and Gels. | 15 questions in 20 minutes | Mock test for JEE preparation | Free important questions MCQ to study for JEE Exam | Download free PDF with solutions
Test: Emulsions and Gels. - Question 1
 white precipitate of
white precipitate of  is peptized with di.
is peptized with di.  . The sol particle will carry
. The sol particle will carry
Detailed Solution for Test: Emulsions and Gels. - Question 1
Test: Emulsions and Gels. - Question 2
The gold numbers of protective colloids  and
and  are
are  and 40 respectively. The protective powers of
and 40 respectively. The protective powers of  and
and  are in the order :
are in the order :
 and
and  are
are  and 40 respectively. The protective powers of
and 40 respectively. The protective powers of  and
and  are in the order :
are in the order :
Detailed Solution for Test: Emulsions and Gels. - Question 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Test: Emulsions and Gels. - Question 3
In an experiment, addition of  of
of 
 to
to  of arsenius sulphide sol just causes the complete coagulation in
of arsenius sulphide sol just causes the complete coagulation in  . The flocculating value of the effective ion is
. The flocculating value of the effective ion is
 of
of 
 to
to  of arsenius sulphide sol just causes the complete coagulation in
of arsenius sulphide sol just causes the complete coagulation in  . The flocculating value of the effective ion is
. The flocculating value of the effective ion is
Detailed Solution for Test: Emulsions and Gels. - Question 3
Test: Emulsions and Gels. - Question 4
Arrange the following electrolytes in the increasing order of coagulating power for ferric hydroxide sol

Detailed Solution for Test: Emulsions and Gels. - Question 4
Detailed Solution for Test: Emulsions and Gels. - Question 5
Test: Emulsions and Gels. - Question 6
Given below are a few electrolytes, indicate which one among them will bring about the coagulation of a gold sol quickest and in the least of concentration?
Detailed Solution for Test: Emulsions and Gels. - Question 6
Test: Emulsions and Gels. - Question 7
Under the influence of an electric field, the particles in a sol migrate towards cathode. The coagulation of the same sol is studied using  ,
,  and
and  solutions. Their coagulating values will be in maximum for:
solutions. Their coagulating values will be in maximum for:
 ,
,  and
and  solutions. Their coagulating values will be in maximum for:
solutions. Their coagulating values will be in maximum for:
Detailed Solution for Test: Emulsions and Gels. - Question 7
Test: Emulsions and Gels. - Question 8
The process of converting a precipitate into colloidal solution is known as
Detailed Solution for Test: Emulsions and Gels. - Question 8
Detailed Solution for Test: Emulsions and Gels. - Question 9
Test: Emulsions and Gels. - Question 10
Coagulation value of the electrolytes  and
and  for
for  sol are
sol are  and 52 respectively. How many times
and 52 respectively. How many times  has greater coagulating power than
has greater coagulating power than  ?
?
 and
and  for
for  sol are
sol are  and 52 respectively. How many times
and 52 respectively. How many times  has greater coagulating power than
has greater coagulating power than  ?
?
Detailed Solution for Test: Emulsions and Gels. - Question 10
Test: Emulsions and Gels. - Question 11
The protecting power of lyophilic colloidal sol is expressed in terms of :
Detailed Solution for Test: Emulsions and Gels. - Question 11
Test: Emulsions and Gels. - Question 12
Which property of colloids is not dependent on the charge of colloidal particles?
Detailed Solution for Test: Emulsions and Gels. - Question 12
Detailed Solution for Test: Emulsions and Gels. - Question 13
Test: Emulsions and Gels. - Question 14
Which of the following is most powerful to coagulate the negative colloid?
Detailed Solution for Test: Emulsions and Gels. - Question 14
Detailed Solution for Test: Emulsions and Gels. - Question 15
Information about Test: Emulsions and Gels. Page
In this test you can find the Exam questions for Test: Emulsions and Gels. solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Emulsions and Gels., EduRev gives you an ample number of Online tests for practice
Download as PDF



 (Positive charge)
(Positive charge) sol is negatively charged owing to preferential adsorption of
sol is negatively charged owing to preferential adsorption of 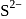 ions. Cation would be the effective ion in coagulation. Flocculating value
ions. Cation would be the effective ion in coagulation. Flocculating value 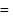 minimum milli mol of the effective ion per litre of sol =
minimum milli mol of the effective ion per litre of sol = 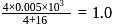
 is most effective for coagulation.
is most effective for coagulation. colloidal solution
colloidal solution precipitate
precipitate sol, the most effective coagulating agent is
sol, the most effective coagulating agent is is a negatively charged sol, therefore positively charged ion is required to coagulate it. Hence,
is a negatively charged sol, therefore positively charged ion is required to coagulate it. Hence,  has the maximum coagulating power.
has the maximum coagulating power.