Test: Phosphorus and Its Compounds - JEE MCQ
Test Description
20 Questions MCQ Test - Test: Phosphorus and Its Compounds
Test: Phosphorus and Its Compounds for JEE 2024 is part of JEE preparation. The Test: Phosphorus and Its Compounds questions and answers have been prepared
according to the JEE exam syllabus.The Test: Phosphorus and Its Compounds MCQs are made for JEE 2024 Exam.
Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Phosphorus and Its Compounds below.
Solutions of Test: Phosphorus and Its Compounds questions in English are available as part of our course for JEE & Test: Phosphorus and Its Compounds solutions in
Hindi for JEE course.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free. Attempt Test: Phosphorus and Its Compounds | 20 questions in 20 minutes | Mock test for JEE preparation | Free important questions MCQ to study for JEE Exam | Download free PDF with solutions
Detailed Solution for Test: Phosphorus and Its Compounds - Question 1
Test: Phosphorus and Its Compounds - Question 2
The number of  bonds and the oxidation state of phosphorus atom in pyrophosphoric acid
bonds and the oxidation state of phosphorus atom in pyrophosphoric acid  respectively are :
respectively are :
 bonds and the oxidation state of phosphorus atom in pyrophosphoric acid
bonds and the oxidation state of phosphorus atom in pyrophosphoric acid  respectively are :
respectively are :
Detailed Solution for Test: Phosphorus and Its Compounds - Question 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Test: Phosphorus and Its Compounds - Question 3
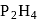 can be removed from phosphine containing traces of it
can be removed from phosphine containing traces of it
Detailed Solution for Test: Phosphorus and Its Compounds - Question 3
Detailed Solution for Test: Phosphorus and Its Compounds - Question 4
Test: Phosphorus and Its Compounds - Question 5
One mole of calcium phosphide on reaction with excess of water gives
Detailed Solution for Test: Phosphorus and Its Compounds - Question 5
Detailed Solution for Test: Phosphorus and Its Compounds - Question 6
Detailed Solution for Test: Phosphorus and Its Compounds - Question 7
Test: Phosphorus and Its Compounds - Question 8
Which of the following is incorrect for white and red phosphorus ?
Detailed Solution for Test: Phosphorus and Its Compounds - Question 8
Test: Phosphorus and Its Compounds - Question 9
Which of the following oxy-acids has the maximum number of hydrogens directly attached to phosphorus?
Detailed Solution for Test: Phosphorus and Its Compounds - Question 9
Test: Phosphorus and Its Compounds - Question 10
The element that can form multiple bonds with itself from the following is
Detailed Solution for Test: Phosphorus and Its Compounds - Question 10
Test: Phosphorus and Its Compounds - Question 11
Which of the following compound has a P-P bond?
Detailed Solution for Test: Phosphorus and Its Compounds - Question 11
Detailed Solution for Test: Phosphorus and Its Compounds - Question 12
Test: Phosphorus and Its Compounds - Question 13
Superphosphate of lime is obtained from the reaction of :
Detailed Solution for Test: Phosphorus and Its Compounds - Question 13
Test: Phosphorus and Its Compounds - Question 14
Phosphine is not obtained by which of the following reaction?
Detailed Solution for Test: Phosphorus and Its Compounds - Question 14
Test: Phosphorus and Its Compounds - Question 15
Phosphorus pentoxide is widely used as ............
Detailed Solution for Test: Phosphorus and Its Compounds - Question 15
Detailed Solution for Test: Phosphorus and Its Compounds - Question 16
Test: Phosphorus and Its Compounds - Question 17
The P − P − P bond angle in white phosphorus is
Detailed Solution for Test: Phosphorus and Its Compounds - Question 17
Detailed Solution for Test: Phosphorus and Its Compounds - Question 18
Test: Phosphorus and Its Compounds - Question 19
The number of P − O − P bonds in cyclic metaphosphate ion is
Detailed Solution for Test: Phosphorus and Its Compounds - Question 19
Test: Phosphorus and Its Compounds - Question 20
Which of the following metaphosphate ion is not known to exist in free state?
Detailed Solution for Test: Phosphorus and Its Compounds - Question 20
Information about Test: Phosphorus and Its Compounds Page
In this test you can find the Exam questions for Test: Phosphorus and Its Compounds solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Phosphorus and Its Compounds, EduRev gives you an ample number of Online tests for practice
Download as PDF


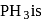 :
: (Lewis base) can react with
(Lewis base) can react with  (Lewis acid).
(Lewis acid).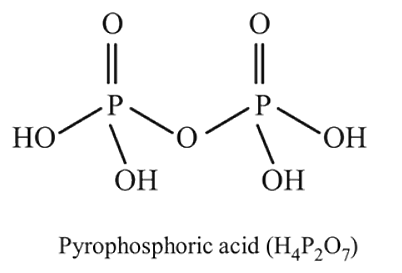
 atom is bound to one oxygen
atom is bound to one oxygen 

 Total
Total 
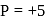
 present condenses and pure
present condenses and pure  is obtained.
is obtained. is absorbed forming
is absorbed forming 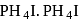 when treated with
when treated with 
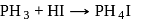
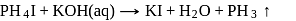
 and
and  , the correct choice is
, the correct choice is is as given below.
is as given below.
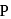 in this acid is
in this acid is 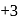 , whereas
, whereas 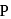 may have
may have  oxidation state also. Therefore,
oxidation state also. Therefore,  can be oxidised which means
can be oxidised which means 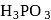 is a reducing agent.
is a reducing agent.
 or
or  It is metaphosphoric acid which is a cyclic phosphate.
It is metaphosphoric acid which is a cyclic phosphate.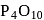 is not used to dry
is not used to dry 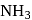 gas because
gas because
 only white phosphorus is soluble in
only white phosphorus is soluble in 

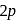 orbitals (lower energy), shows
orbitals (lower energy), shows  bonding. Whereas, in the heavier elements the overlapping orbitals are bigger and are very high in energy as atomic size increases down the group. Thus, in these cases, effective overlapping for
bonding. Whereas, in the heavier elements the overlapping orbitals are bigger and are very high in energy as atomic size increases down the group. Thus, in these cases, effective overlapping for  bond, does not takes place.
bond, does not takes place.
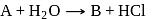

 and
and 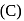 will be respectively
will be respectively
 does not react with
does not react with 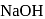 to give
to give  .
.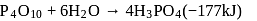
 is heated with
is heated with  to form :
to form :

















