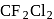Test: Noble gases and its properties - JEE MCQ
Test Description
15 Questions MCQ Test - Test: Noble gases and its properties
Test: Noble gases and its properties for JEE 2024 is part of JEE preparation. The Test: Noble gases and its properties questions and answers have been prepared
according to the JEE exam syllabus.The Test: Noble gases and its properties MCQs are made for JEE 2024 Exam.
Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Noble gases and its properties below.
Solutions of Test: Noble gases and its properties questions in English are available as part of our course for JEE & Test: Noble gases and its properties solutions in
Hindi for JEE course.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free. Attempt Test: Noble gases and its properties | 15 questions in 20 minutes | Mock test for JEE preparation | Free important questions MCQ to study for JEE Exam | Download free PDF with solutions
Test: Noble gases and its properties - Question 1
Compounds formed when the noble gases get entrapped in the cavities of crystal lattices of certain organic and inorganic compounds are known as
Detailed Solution for Test: Noble gases and its properties - Question 1
Test: Noble gases and its properties - Question 2
Ionisation potential values of noble gases decrease down the group with increase in atomic size. Xenon forms binary fluorides by the direct reaction of elements. Identify the correct statement(s) from below.
Detailed Solution for Test: Noble gases and its properties - Question 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Detailed Solution for Test: Noble gases and its properties - Question 3
Test: Noble gases and its properties - Question 4
Incorrect statement regarding following reactions is:


Detailed Solution for Test: Noble gases and its properties - Question 4
Test: Noble gases and its properties - Question 5
MF + XeF4 ⟶ ′A′(M+ = Alkali metal cation)
The state of hybridisation of the central atomin 'A' and shape of the species are:
Detailed Solution for Test: Noble gases and its properties - Question 5
Detailed Solution for Test: Noble gases and its properties - Question 6
Detailed Solution for Test: Noble gases and its properties - Question 7
Test: Noble gases and its properties - Question 8
When helium gas is allowed to expand into vaccum, heating effect is observed. The reason for this is (assume He as a non ideal gas)
Detailed Solution for Test: Noble gases and its properties - Question 8
Detailed Solution for Test: Noble gases and its properties - Question 9
Test: Noble gases and its properties - Question 10
The ionization energy of 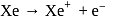 is very close to that of
is very close to that of
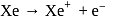 is very close to that of
is very close to that of
Detailed Solution for Test: Noble gases and its properties - Question 10
Test: Noble gases and its properties - Question 11
When a mixture of  and
and  in the ratio of
in the ratio of  is heated at
is heated at  in a sealed nickel vessel, the compound formed is
in a sealed nickel vessel, the compound formed is
 and
and  in the ratio of
in the ratio of  is heated at
is heated at  in a sealed nickel vessel, the compound formed is
in a sealed nickel vessel, the compound formed is
Detailed Solution for Test: Noble gases and its properties - Question 11
Detailed Solution for Test: Noble gases and its properties - Question 12
Detailed Solution for Test: Noble gases and its properties - Question 13
Test: Noble gases and its properties - Question 14
Which of the following statements is incorrect?
Detailed Solution for Test: Noble gases and its properties - Question 14
Detailed Solution for Test: Noble gases and its properties - Question 15
Information about Test: Noble gases and its properties Page
In this test you can find the Exam questions for Test: Noble gases and its properties solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Noble gases and its properties, EduRev gives you an ample number of Online tests for practice
Download as PDF


 :
: 
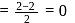
 . No, it is not an oxyacid of
. No, it is not an oxyacid of 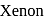
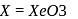 . Yes, it is explosive
. Yes, it is explosive , pentagonal planar
, pentagonal planar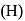 . Tos atomic weight is
. Tos atomic weight is  . But among the mentioned gases
. But among the mentioned gases .
.
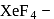 This compound is formed by xenon.
This compound is formed by xenon.

 in
in 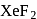 is
is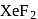 has
has



 is
is
 molecule has a trigonal bipyramidal structure.
molecule has a trigonal bipyramidal structure. is
is