Test: F-Block elements - JEE MCQ
15 Questions MCQ Test - Test: F-Block elements
Statement I : Many trivalent lanthanide ions are coloured both in solid state and in aqueous solution.
Statement II : Colour of these ions is due to the presence of f-electrons.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The number f-electrons in +3 oxidation state of gadolinium  is
is  and in +2 oxidation state of Ytterbium
and in +2 oxidation state of Ytterbium  is
is  . The sum of
. The sum of  and
and 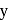 is
is
 is
is  and in +2 oxidation state of Ytterbium
and in +2 oxidation state of Ytterbium  is
is  . The sum of
. The sum of  and
and 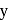 is
isWhich one of the following elements shows maximum number of different oxidation states in its compounds?
Which of the following pairs is expected to form colourless compound?
Gadolinium (atomic number = 64) is a member of 4f series. It's electronic configuration in +3 oxidation state is [Xe] 4f7. What is the ground state electronic configuration of gadolinium?
Which of the following trivalent ion has the largest atomic radii in the lanthanide series
From the list given below, the number of lanthanides which exhibit +4 state in their oxides is Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy



 is less stable than
is less stable than  and thus it will readily accept
and thus it will readily accept  convert into
convert into  and so it will less basic than
and so it will less basic than  .
. is most basic among all given options.
is most basic among all given options.
 is
is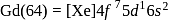
 No. of unpaired electrons
No. of unpaired electrons 
 , oxidation state, while Eu shows oxidation state of
, oxidation state, while Eu shows oxidation state of 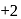 and
and  . Am shows
. Am shows 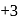 ,
,  and
and  oxidation states. Therefore Americium (Am) has maximum number of oxidation states.
oxidation states. Therefore Americium (Am) has maximum number of oxidation states.
 is (4f)" while that of
is (4f)" while that of  is
is  . They are expected to be colomicis.
. They are expected to be colomicis.

 block elements because
block elements because ". and deu to electronic configuration.
". and deu to electronic configuration. has tendency to go over to
has tendency to go over to  and hence acts as oxidiring agent.
and hence acts as oxidiring agent. being smallest (due to lanthanide contraction) has maximum tendency to form complexes.
being smallest (due to lanthanide contraction) has maximum tendency to form complexes.
 (trivalent lanthanides ions) have E.C.
(trivalent lanthanides ions) have E.C.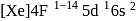
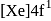 to
to 















