Test: Preparation & Reaction of Phenols - JEE MCQ
15 Questions MCQ Test - Test: Preparation & Reaction of Phenols
On heating aqueous solution of benzene diazonium chloride, which of the following is formed?
For the reaction

The correct statement regarding above reaction is

The correct statement regarding above reaction is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The conversion of O-acylated phenol in presence of  to
to  -acylated phenol is an example for this type of organic reaction
-acylated phenol is an example for this type of organic reaction
 to
to  -acylated phenol is an example for this type of organic reaction
-acylated phenol is an example for this type of organic reactionIdentify the product in the following reaction.

What amount of bromine will be required to convert  of phenol into
of phenol into  -tribromophenol?
-tribromophenol?
An optically active alcohol of formula  produced the following compound when refluxed with
produced the following compound when refluxed with 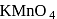

The original compound showed these properties also:

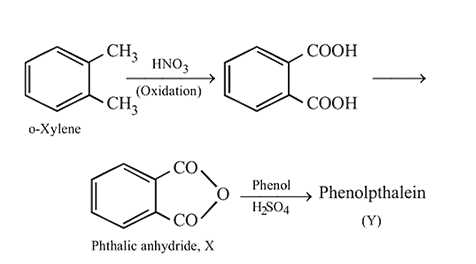
What is structure of  ?
?
Which of the following reactions will not result in the formation of anisole?
Which of the following compound can react with hydroxylamine?
The products formed in the reaction of phenol with  dissolved in
dissolved in 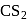 at
at 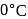 are
are
Identify the nature of product in the following reaction

Phenol can be distinguished from alcohol with
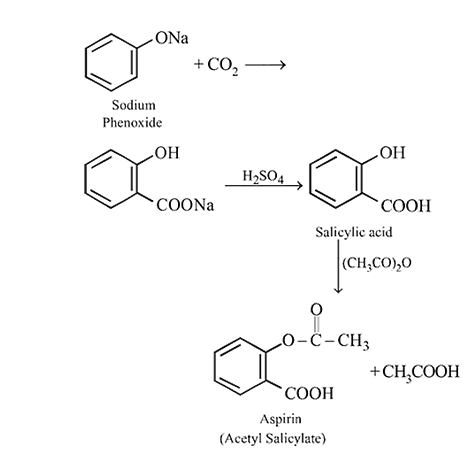
Sodium phenoxide when heated with  under pressure at
under pressure at  yields a product which on acetylation produces
yields a product which on acetylation produces 

The major product  would be
would be
Which one of the following substituents at paraposition is most effective in stabilizing the phenoxide

ion?
1-Phenylethanol can be prepared by reaction of benzaldehyde with









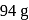 of phenol require
of phenol require 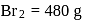
 of phenol require
of phenol require 

 to give
to give  , and not anisole
, and not anisole

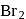 in the presence of non-polar solvent
in the presence of non-polar solvent  , it gives only o- and
, it gives only o- and  -bromophenols instead the trisubstituted products. Reason for the above observation is supression of phenoxide ion in non-polar solvent. Thus, we get only mono substituted products.
-bromophenols instead the trisubstituted products. Reason for the above observation is supression of phenoxide ion in non-polar solvent. Thus, we get only mono substituted products.
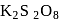 is an example of Elbs persulphate oxidation.
is an example of Elbs persulphate oxidation.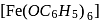 Phenol gives violet colour with neutral
Phenol gives violet colour with neutral 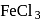

 and
and  are electron donating group and hence decrease the stability of benzene ring
are electron donating group and hence decrease the stability of benzene ring  is weaker electron withdrawing group than
is weaker electron withdrawing group than  . Hence
. Hence  group more stabilize the phenoxide ion at
group more stabilize the phenoxide ion at  -position.
-position.
 alcohol and can be prepared by the reaction of benzaldehyde with Grignard reagent
alcohol and can be prepared by the reaction of benzaldehyde with Grignard reagent  .
.














