Test: Method of preparation for Aldehydes & Ketones - JEE MCQ
Test Description
15 Questions MCQ Test - Test: Method of preparation for Aldehydes & Ketones
Test: Method of preparation for Aldehydes & Ketones for JEE 2024 is part of JEE preparation. The Test: Method of preparation for Aldehydes & Ketones questions and answers have been prepared
according to the JEE exam syllabus.The Test: Method of preparation for Aldehydes & Ketones MCQs are made for JEE 2024 Exam.
Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Method of preparation for Aldehydes & Ketones below.
Solutions of Test: Method of preparation for Aldehydes & Ketones questions in English are available as part of our course for JEE & Test: Method of preparation for Aldehydes & Ketones solutions in
Hindi for JEE course.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free. Attempt Test: Method of preparation for Aldehydes & Ketones | 15 questions in 20 minutes | Mock test for JEE preparation | Free important questions MCQ to study for JEE Exam | Download free PDF with solutions
Test: Method of preparation for Aldehydes & Ketones - Question 1
Rosenmund reduction is used for the preparation of
Detailed Solution for Test: Method of preparation for Aldehydes & Ketones - Question 1
Test: Method of preparation for Aldehydes & Ketones - Question 2
Reduction of acetyl chloride with  in presence of
in presence of  and
and 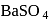 gives:
gives:
Detailed Solution for Test: Method of preparation for Aldehydes & Ketones - Question 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Test: Method of preparation for Aldehydes & Ketones - Question 3
Catalyst  is used in which of the following method of synthesis of aldehyde?
is used in which of the following method of synthesis of aldehyde?
Detailed Solution for Test: Method of preparation for Aldehydes & Ketones - Question 3
Test: Method of preparation for Aldehydes & Ketones - Question 4
The incorrect statement regarding oxo process for synthesis of an aldehyde is
Detailed Solution for Test: Method of preparation for Aldehydes & Ketones - Question 4
Test: Method of preparation for Aldehydes & Ketones - Question 5
Which of the following compounds give a ketone with grignard reagent?
Detailed Solution for Test: Method of preparation for Aldehydes & Ketones - Question 5
Test: Method of preparation for Aldehydes & Ketones - Question 6
Acetyl chloride reacts with ____ to give butan-2-one.
Detailed Solution for Test: Method of preparation for Aldehydes & Ketones - Question 6
Test: Method of preparation for Aldehydes & Ketones - Question 7
Dialkyl cadmium reacts with a compound to form a ketone. The compound is:
Detailed Solution for Test: Method of preparation for Aldehydes & Ketones - Question 7
Test: Method of preparation for Aldehydes & Ketones - Question 8
An acetoacetic ester synthesis of a ketone proceeds by alkylation of the enolate of the acetoacetic ester followed by ester hydrolysis and decarboxylation of the 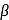 -ketoacid. Which of the following methyl ketones is difficult to prepare by this method?
-ketoacid. Which of the following methyl ketones is difficult to prepare by this method?
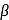 -ketoacid. Which of the following methyl ketones is difficult to prepare by this method?
-ketoacid. Which of the following methyl ketones is difficult to prepare by this method?
Detailed Solution for Test: Method of preparation for Aldehydes & Ketones - Question 8
Detailed Solution for Test: Method of preparation for Aldehydes & Ketones - Question 9
Test: Method of preparation for Aldehydes & Ketones - Question 10
The correct set of reagents to carry out the following conversion

Detailed Solution for Test: Method of preparation for Aldehydes & Ketones - Question 10
Test: Method of preparation for Aldehydes & Ketones - Question 11
What is  in the following conversion?
in the following conversion?

 in the following conversion?
in the following conversion?
Detailed Solution for Test: Method of preparation for Aldehydes & Ketones - Question 11
Test: Method of preparation for Aldehydes & Ketones - Question 12
Methyl ethyl ketone is prepared by the oxidation of
Detailed Solution for Test: Method of preparation for Aldehydes & Ketones - Question 12
Test: Method of preparation for Aldehydes & Ketones - Question 13
Which alkene on ozonolysis gives  and
and  ?
?
Detailed Solution for Test: Method of preparation for Aldehydes & Ketones - Question 13
Detailed Solution for Test: Method of preparation for Aldehydes & Ketones - Question 14
Test: Method of preparation for Aldehydes & Ketones - Question 15
5 - Methyl - 2 -hexanone can be synthesised from acetoacetic ester and RX. Which of the following RX is used?
Detailed Solution for Test: Method of preparation for Aldehydes & Ketones - Question 15
Information about Test: Method of preparation for Aldehydes & Ketones Page
In this test you can find the Exam questions for Test: Method of preparation for Aldehydes & Ketones solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Method of preparation for Aldehydes & Ketones, EduRev gives you an ample number of Online tests for practice
Download as PDF



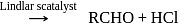

 from nitriles
from nitriles  using
using  chloride
chloride 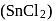 , hydrochloric acid
, hydrochloric acid  and quenching the resulting iminium salt ([R
and quenching the resulting iminium salt ([R ) with water
) with water  .
.


 reaction, and sterically hindered alkyl groups are difficult to introduce. However, even dialkylation is possible with reactive alkylating agents, as in (d).
reaction, and sterically hindered alkyl groups are difficult to introduce. However, even dialkylation is possible with reactive alkylating agents, as in (d).
 is
is





















