Test: Preparation and Reactions of Glucose - JEE MCQ
Test Description
15 Questions MCQ Test - Test: Preparation and Reactions of Glucose
Test: Preparation and Reactions of Glucose for JEE 2024 is part of JEE preparation. The Test: Preparation and Reactions of Glucose questions and answers have been prepared
according to the JEE exam syllabus.The Test: Preparation and Reactions of Glucose MCQs are made for JEE 2024 Exam.
Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Preparation and Reactions of Glucose below.
Solutions of Test: Preparation and Reactions of Glucose questions in English are available as part of our course for JEE & Test: Preparation and Reactions of Glucose solutions in
Hindi for JEE course.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free. Attempt Test: Preparation and Reactions of Glucose | 15 questions in 20 minutes | Mock test for JEE preparation | Free important questions MCQ to study for JEE Exam | Download free PDF with solutions
Test: Preparation and Reactions of Glucose - Question 1
Glucose molecule reacts with 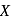 number of molecules of phenylhydrazine to yield osazone. The value of
number of molecules of phenylhydrazine to yield osazone. The value of 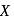 is
is
Detailed Solution for Test: Preparation and Reactions of Glucose - Question 1
Test: Preparation and Reactions of Glucose - Question 2
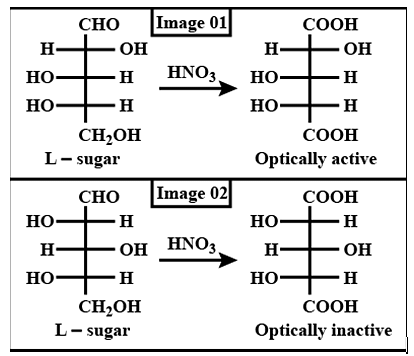
Which L-sugar on oxidation gives an optically active dibasic acid ( 2 COOH groups)?
Detailed Solution for Test: Preparation and Reactions of Glucose - Question 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Test: Preparation and Reactions of Glucose - Question 3
In cells the net production of ATP molecules generated from one glucose molecule is
Detailed Solution for Test: Preparation and Reactions of Glucose - Question 3
Test: Preparation and Reactions of Glucose - Question 4
Hydrolysis of sucrose with dilute aqueous sulphuric acid yields
Detailed Solution for Test: Preparation and Reactions of Glucose - Question 4
Test: Preparation and Reactions of Glucose - Question 5
In osazone formation, glucose reacts with three molecules of phenylhydrazine. Which statement is true regarding this?
Detailed Solution for Test: Preparation and Reactions of Glucose - Question 5
Test: Preparation and Reactions of Glucose - Question 6
What will happen when 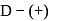 -glucose is treated with methanolic
-glucose is treated with methanolic 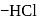 followed by Tollens' reagent?
followed by Tollens' reagent?
Detailed Solution for Test: Preparation and Reactions of Glucose - Question 6
Test: Preparation and Reactions of Glucose - Question 7
Among the three compounds shown below, two yield the same product on reaction with warm 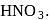 The exception is:
The exception is:
Detailed Solution for Test: Preparation and Reactions of Glucose - Question 7
Test: Preparation and Reactions of Glucose - Question 8
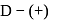 -Glucose
-Glucose 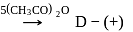 -Glucose pentaacetate
-Glucose pentaacetate Which statement is true about glucose pentaacetate?
Detailed Solution for Test: Preparation and Reactions of Glucose - Question 8
Test: Preparation and Reactions of Glucose - Question 9
The two forms of D-glucopyranose obtained from the solution of D-glucose are called
Detailed Solution for Test: Preparation and Reactions of Glucose - Question 9
Test: Preparation and Reactions of Glucose - Question 10
The number of molecules of ATP produced per molecule of glucose in glycolysis is
Detailed Solution for Test: Preparation and Reactions of Glucose - Question 10
Test: Preparation and Reactions of Glucose - Question 11
Reducing saccharides among the following are
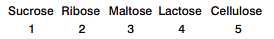
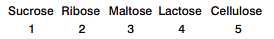
Detailed Solution for Test: Preparation and Reactions of Glucose - Question 11
Test: Preparation and Reactions of Glucose - Question 12
Carbohydrates are stored in plants and animals in which of the following forms respectively?
Detailed Solution for Test: Preparation and Reactions of Glucose - Question 12
Test: Preparation and Reactions of Glucose - Question 13
Which of the following statements are true about carbohydrates?
(i) Monosaccharides can be hydrolysed.
(ii) The two monosaccharide units obtained on hydrolysis of a disaccharide can either be same or different.
(iii) Polysaccharides are now sweet in taste.
(iv) All monosaccharides are not reducing. sugars.
(i) Monosaccharides can be hydrolysed.
(ii) The two monosaccharide units obtained on hydrolysis of a disaccharide can either be same or different.
(iii) Polysaccharides are now sweet in taste.
(iv) All monosaccharides are not reducing. sugars.
Detailed Solution for Test: Preparation and Reactions of Glucose - Question 13
Test: Preparation and Reactions of Glucose - Question 14
Which of the following does not reduce Benedict's solution?
Detailed Solution for Test: Preparation and Reactions of Glucose - Question 14
Detailed Solution for Test: Preparation and Reactions of Glucose - Question 15
Information about Test: Preparation and Reactions of Glucose Page
In this test you can find the Exam questions for Test: Preparation and Reactions of Glucose solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Preparation and Reactions of Glucose , EduRev gives you an ample number of Online tests for practice
Download as PDF


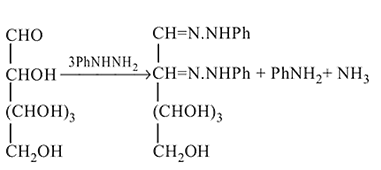
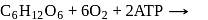
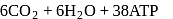
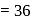
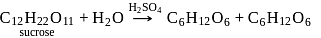
 in fructose) group. Second molecule of the reagent oxidizes
in fructose) group. Second molecule of the reagent oxidizes 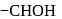 - at position 2 (in aldoses) or
- at position 2 (in aldoses) or 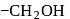 at position 1 (in ketoses) to form
at position 1 (in ketoses) to form 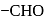 or
or 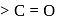 respectively. The third molecule again undergoes nucleophilic addition on the newly developed carbonyl group to form osazone.
respectively. The third molecule again undergoes nucleophilic addition on the newly developed carbonyl group to form osazone.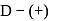 - glucose is treated with methanolic
- glucose is treated with methanolic 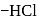 followed by Tollens' reagent, no characteristic colour or ppt. will be formed.
followed by Tollens' reagent, no characteristic colour or ppt. will be formed.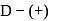 glucose with methanolic
glucose with methanolic 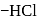 leads to formation of methyl glucoside
leads to formation of methyl glucoside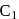 of hemiacetal is methylated to form acetal). Acetal, is not hydrolysable by base, so it will not respond Tollen's reagent.
of hemiacetal is methylated to form acetal). Acetal, is not hydrolysable by base, so it will not respond Tollen's reagent.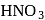 oxidation of aldehyde and alcohol takes place which gives carboxylic acid.
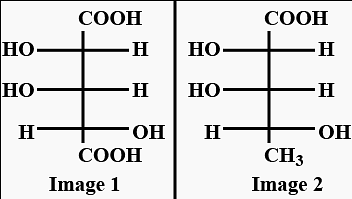
oxidation of aldehyde and alcohol takes place which gives carboxylic acid.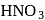 . Thus (a)
. Thus (a) 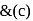 will give same product i.e.
will give same product i.e.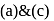 .
.
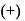 -glucose, it is the
-glucose, it is the 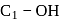 of the hemiacetal that is acetylated and not the
of the hemiacetal that is acetylated and not the 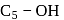 that forms the ring (cyclic structure). Since equilibrium with the open-chain aldehyde is prevented, the penta-acetate does not respond the aldehydic reactions.
that forms the ring (cyclic structure). Since equilibrium with the open-chain aldehyde is prevented, the penta-acetate does not respond the aldehydic reactions.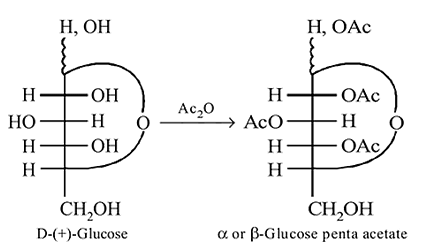
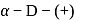 -glucopyranose and
-glucopyranose and 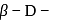 (+)-glucopyranose. These are anomers (a pair of stereoisomers) which differ in configuration only around first carbon atom are called anomers.
(+)-glucopyranose. These are anomers (a pair of stereoisomers) which differ in configuration only around first carbon atom are called anomers.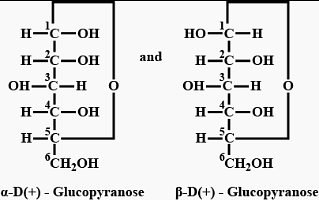
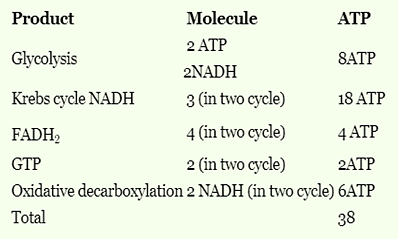
 Glucose + Fructose
Glucose + Fructose Glucose + Glucose
Glucose + Glucose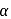 -hydroxy ketonic group, which is also reducing group (different from ordinary ketonic group)
-hydroxy ketonic group, which is also reducing group (different from ordinary ketonic group)














