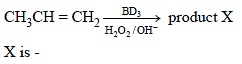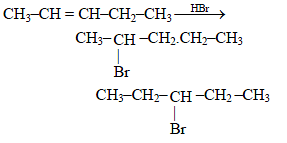Hydrocarbon - 2 - JEE MCQ
30 Questions MCQ Test - Hydrocarbon - 2
The product(s) obtained via oxymercuation (HgSO4 + H2SO4) of 1-butyne would be -
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The synthesis of 3-octyne is achieved by adding a bromoalkane into a mixture of sodium amide and an alkyne. The bromoalkane and alkyne respectively are -
Identify a reagent from the following list which can easily distinguish between 1-butyne and 2-butyne
Acetylene may be prepared using Kolbe's electrolytic method employing -
Assertion : Addition of HBr to 1-butene gives two optical isomers.
Reason : The product contains one asymmetric carbon.
The product of reaction between one mole of acetylene and two mole of HCHO in the presence of Cu2Cl2 -
Consider the following reactions -

Identify the structure of the major product 'X'
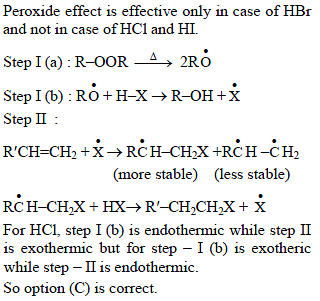
In the presence of peroxide, hydrogen chloride and hydrogen iodide do not give anti- Markovnikov addition to alkene because -

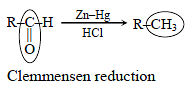
Hydrogenation of the above compound in the presence of poisoned pallodium catalyst gives
Acetylene may be prepared using Kolbe's electrolytic method employing -
The number of structural and configurational isomers of a bromo compound, C5H9Br formed by the addition of HBr to 2-pentyne respectively -