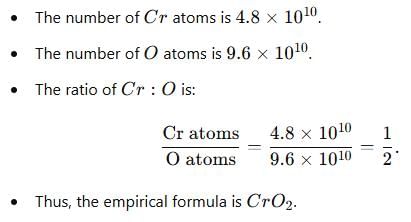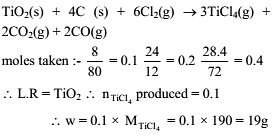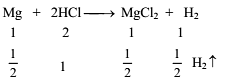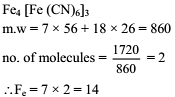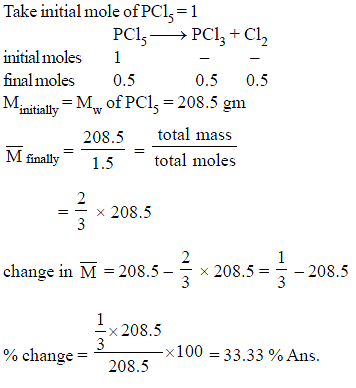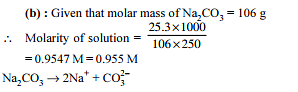Some Basic Concepts of Chemistry - 2 - JEE MCQ
30 Questions MCQ Test - Some Basic Concepts of Chemistry - 2
The volume of 1.204 × 1024 molecules of water at 4ºC is …………… (ml)
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
There are two common oxides of Sulphur, one of which contains 50% O2 by weight, the other almost exactly 60%. The weights of sulphur
which combine with 1 g of O2 (fixed) are in the ratio of -
which combine with 1 g of O2 (fixed) are in the ratio of -
In an ionic compound moles ratio of cation to anion is 1 : 2. If atomic masses of metal and non-metal respectively are 138 and 19, then
correct statement is -
The number of atoms of Cr and O are 4.8 × 1010 and 9.6 × 1010 respectively. Its empirical formula is -
Iron forms two oxides, in first oxide 56 gram. Iron is found to be combined with 16 gram oxygen and in second oxide 112 gram iron is
found to be combined with 48 gram oxygen. This data satisfy the law of -
0.2 mole of HCl and 0.1 mole of barium chloride were dissolved in water to produce a 500-mL solution. The molarity of the Cl– ions is -
A vessel contains 8 gram TiO2, 2.4 gram carbon and 28.4 gram Cl2. Maximum mass of TiCl4 which can be produced is -
3TiO2(s) + 4C(s) + 6Cl2(g) → 3TiCl4(g) + 2CO2(g) + 2CO(g)
(Consider reaction goes to completion ; atomic mass of Ti = 48)
The mole fraction of a given sample of I2 in C6H6 is 0.2. The molality of I2 in C6H6 is -
The number of molecules present in 88 g of CO2 (Relative molecular mass of CO2 = 44)
When 20 ml of pure acetic acid (density = 0.75 gm ml–1) is mixed with 50 gm of water (density = 1gm ml–1) at a certain temperature. Calculate the molality of acetic acid in the final solution.
If NA is Avogadro number, then the number of valence electrons in 4.2 g of N3– ions is -
12g of Mg (atm. mass 24) will react completely with acid to give -
The iron atoms in 1720 amu of ferric ferrocyanide is .....................
When 20 ml of pure acetic acid (density = 0.75 gm ml–1) is mixed with 50 gm of water (density = 1gm ml–1) at a certain temperature. Calculate the molality of acetic acid in the final solution.
Calculate percentage change in Mavg of the mixture, if PCl5 undergo 50% decomposition.
PCl5 → PCl3 + Cl2
Molarity of aqueous NaOH solution will be, if mole fraction of NaOH in the solution is 0.5. [Given : density of pure NaOH = 4 gm/ml]
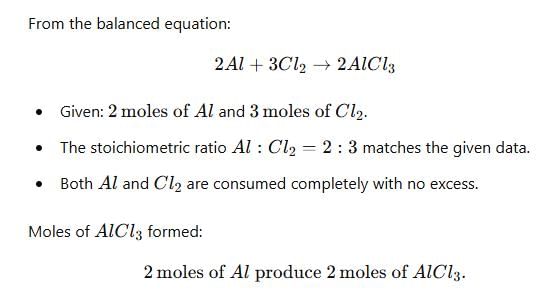
2 moles of Al react with 3 moles of Cl2 to form AlCl3. What will be the limiting reagent, and how many moles of AlCl3 will be formed?
A compound contains 50% sulfur and 50% oxygen by mass. What is the empirical formula of the compound?
A sample contains 3 mol of N2 and 2mol of H2. What is the total number of molecules in the sample?