Jharkhand TGT (JSSC) Mock Test - 3 - Jharkhand (JSSC) PRT/TGT MCQ
30 Questions MCQ Test - Jharkhand TGT (JSSC) Mock Test - 3
If a line makes the angles 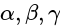 with the axes, then the value of
with the axes, then the value of 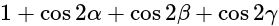 is equal to:
is equal to:
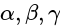 with the axes, then the value of
with the axes, then the value of 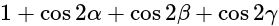 is equal to:
is equal to:| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
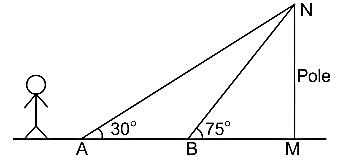
A person walking along a straight road observes that at two points 1 km apart, the angle of elevation of a pole in front of the person is 30° and 75°. The height of the pole is:
A cylindrical vessel of a radius of 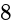 cm contains water. A solid sphere of radius
cm contains water. A solid sphere of radius 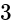 cm is dipped into the water until it is completely immersed. The water level in the vessel will rise by?
cm is dipped into the water until it is completely immersed. The water level in the vessel will rise by?
On increasing the length of wire by four times and increasing the radius of wire by 'X' times the resistance of wire remains unchanged then calculate the value of 'X'.
In a Wien-bridge oscillator, if the resistances in the positive feedback circuit are decreased, the frequency:
Stefan Boltzmann law is applicable for heat transfer by __________.
________ is the thermodynamics process in which no heat flows between the system and surrounding.
A convex lens of focal length 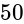 cm and a concave lens of focal length
cm and a concave lens of focal length 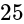 cm are placed in contact with each other. Then the equivalent focal length of the combination will be:
cm are placed in contact with each other. Then the equivalent focal length of the combination will be:
 Identical cells are joined in series with two cells
Identical cells are joined in series with two cells 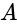 and
and 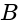 with reversed polarities. e.m.f. of each cell is
with reversed polarities. e.m.f. of each cell is 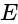 and the internal resistance is
and the internal resistance is 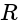 . Potential difference across the cell
. Potential difference across the cell 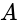 and
and 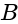 is :
is : 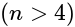
Which of the following statements about electric field lines associated with electric charges is false?
A short bar magnet placed with its axis at 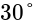 with an external field of
with an external field of 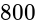 G experiences a torque of
G experiences a torque of 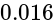 Nm. What is the magnetic moment of the magnet ?
Nm. What is the magnetic moment of the magnet ?
Kirchoff's current law is based on conservation of ______ while Kirchoff's voltage law is based on conservation of _________.
The ratio of magnetic field and magnetic moment at the centre of a current carrying circular loop is X. When both the current and radius is doubled the ratio will be
A positive charge q is placed at the center of a spherical conducting shell. What is the electric field inside the shell?
The cooking method by which Idli is cooked is:
The electrons, identified by quantum numbers 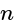 and
and 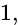 (i)
(i) 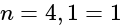 , (ii)
, (ii) 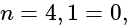 (iii)
(iii) 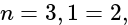 and (iv)
and (iv) 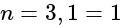 can be placed in order of increasing energy, from the lowest to highest, as:
can be placed in order of increasing energy, from the lowest to highest, as:
Direction: Choose the correct answer among the alternatives given.
One mole of ethyl acetate on treatment with an excess of 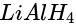 in dry ether and subsequent acidification produces.
in dry ether and subsequent acidification produces.
The equilibrium constant of the reaction:
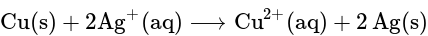
 at
at 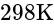 is:
is:
Rutherford's α-particle experiment showed that the atoms have:
Three complexes,
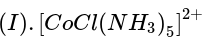

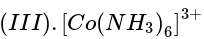
absorb light in the visible region. The correct order of the wavelength of light absorbed by them is:
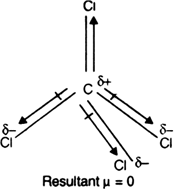
Carbon tetrachloride has zero dipole moment because of ________.
In the Mendeleev periodic table, gaps were left for the undiscovered elements. Which of the following elements found a place in the periodic table later?


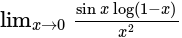 equal to?
equal to?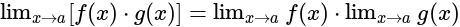
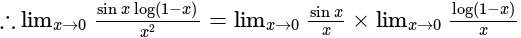
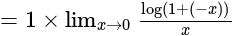
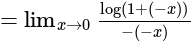
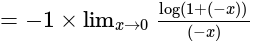

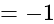
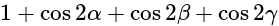
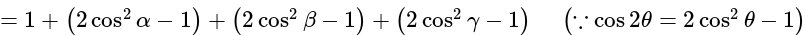
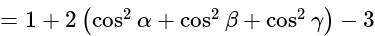
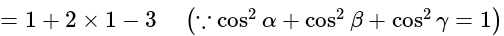
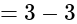
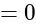
 The value of
The value of 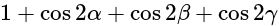 is
is 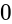 .
.
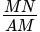

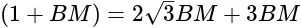
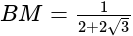
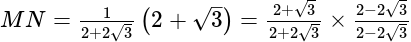
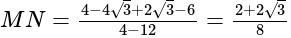
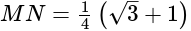 km = 250
km = 250 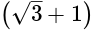 m.
m.
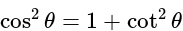 .
.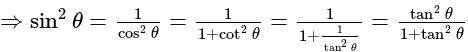
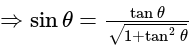
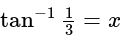 and
and 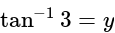
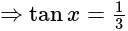 and
and 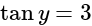
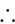 The given expression
The given expression 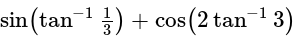 can be written as:
can be written as: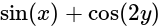
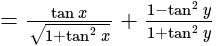
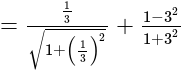
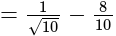
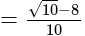
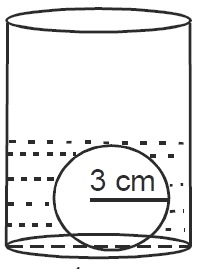
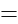 The volume of the sphere
The volume of the sphere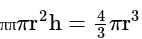
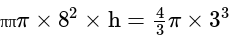
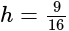
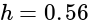
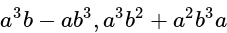 and
and 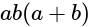 ?
?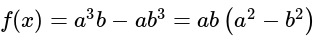

 LCM of
LCM of 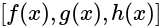
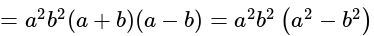
 cm (convex lens)
cm (convex lens) cm (concave lens)
cm (concave lens)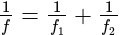
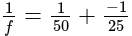
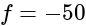 cm
cm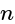 cells are connected in series, hence their e.m.f. adds up.
cells are connected in series, hence their e.m.f. adds up.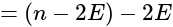
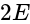 corresponds to emf of
corresponds to emf of 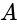 and
and 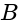 having opposite polarity.
having opposite polarity.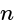 cells are connected each having
cells are connected each having 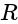 internal resistance, total resistance in the circuit, is:
internal resistance, total resistance in the circuit, is: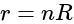
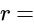 Total internal resistances
Total internal resistances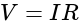
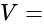 Potential Difference
Potential Difference 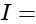 Current
Current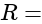 Resistance
Resistance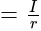
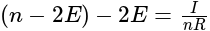
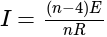
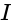 is the current flowing through the circuit.
is the current flowing through the circuit.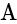 and
and 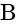 =
= 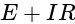
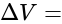 Potential difference across
Potential difference across 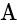 and
and 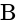
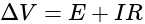
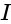 , we get
, we get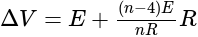
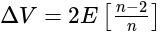

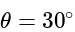
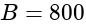 G
G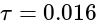 Nm
Nm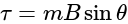
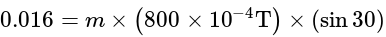
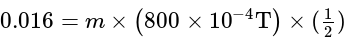
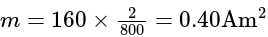
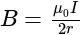 ....(i)
....(i)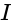 =current
=current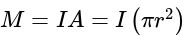 .....(ii)
.....(ii) 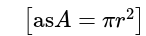
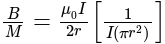
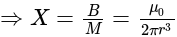
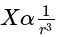
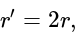 then the new ratio is
then the new ratio is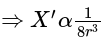
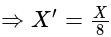
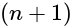 , the greater is the energy of orbitals.
, the greater is the energy of orbitals.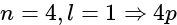 orbital
orbital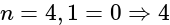 s orbital
s orbital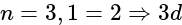 orbital
orbital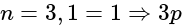 orbital
orbital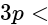 (ii)
(ii)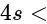 (iii)
(iii)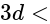 (i)
(i)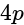
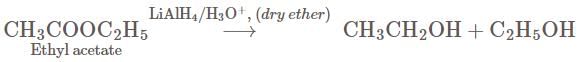
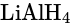 gives 2 moles of Ethyl Alcohol.
gives 2 moles of Ethyl Alcohol. ,
, cell
cell 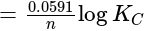
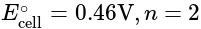
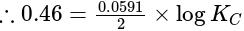
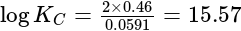
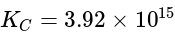
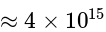
 -D-glucopyranosyl-(
-D-glucopyranosyl-(  -
- 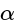 -D-glucopyranoside units. While inulin is polysaccharides, raffinose is trisaccharide and cellulose is polysaccharides.
-D-glucopyranoside units. While inulin is polysaccharides, raffinose is trisaccharide and cellulose is polysaccharides.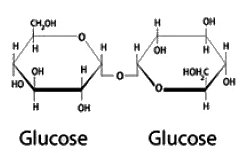
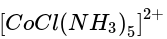 has the weakest field and therefore will absorb light of least energy and highest wavelength.
has the weakest field and therefore will absorb light of least energy and highest wavelength. has a field weaker than the above compound and therefore absorb radiation of lesser energy and more wavelength.
has a field weaker than the above compound and therefore absorb radiation of lesser energy and more wavelength.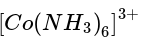 will absorb radiation of highest energy and least wavelength.
will absorb radiation of highest energy and least wavelength. .
.
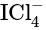 contains 4 bond pairs of electrons and 2 lone pairs of electrons. The central iodine atom undergoes
contains 4 bond pairs of electrons and 2 lone pairs of electrons. The central iodine atom undergoes 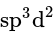 hybridization. The expected geometry is octahedral and the actual geometry is square planar.
hybridization. The expected geometry is octahedral and the actual geometry is square planar. 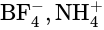 and
and 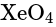 are tetrahedral in shape.
are tetrahedral in shape.














