Test: Nernst Equation ,Conductance of Electrolytic Solutions(11 Oct) - JEE MCQ
10 Questions MCQ Test - Test: Nernst Equation ,Conductance of Electrolytic Solutions(11 Oct)
Gibbs free energy change for a cell reaction is positive what does it indicates?
In the equation, ΔG° = – nF E° cell ; F is:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Consider the cell reaction:
Cd(s) | Cd2+ (1.0 M) || Cu2+ (1.0 m) | Cu (s)
If we wish to make a cell with more positive voltage using the same substances, we should:
Cd(s) | Cd2+ (1.0 M) || Cu2+ (1.0 m) | Cu (s)
If we wish to make a cell with more positive voltage using the same substances, we should:
Conductivity (K) of 0.01 M NaCI solution is 0.00145 Scm-1. What happens to the conductivity if extra 100 mL of H2O be added to the above solution?
Specific conductance of 0.01 N KCI solution is x Scm-1 having conductance y S. Thus, specific conductance of 0.01 N NaCI having conductance zS is (in S cm-1)
500 mL of an aqueous solution contains 0.1 mole of KCl. If its specific conductance is x Scm-1, its molar conductance will be (in Scm2 mol-1)
Which quantity is temperature independent?
Given, (Scm2 mol-1)for different electrolytes
Thus, of CH3COOH is
 is reduced by electrolysis at low potentials and high currents. If
is reduced by electrolysis at low potentials and high currents. If  amperes of current is passed through molten
amperes of current is passed through molten  for 6 hours, what mass of aluminium is produced? (Assume
for 6 hours, what mass of aluminium is produced? (Assume  current efficiency. At. mass of
current efficiency. At. mass of  )
)


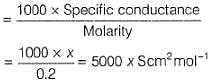
 or
or 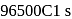 the charge carried by
the charge carried by  of electrons when water is electrolysed
of electrons when water is electrolysed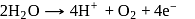
 of
of  Thus 1 Faraday of electricity liberate
Thus 1 Faraday of electricity liberate of
of  of
of 
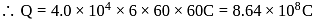
 of
of 

 of
of 















