Test: SN1 & SN2 Reactions(17 Nov) - JEE MCQ
10 Questions MCQ Test - Test: SN1 & SN2 Reactions(17 Nov)
Which of the following can catalyse the following SN1 reaction?
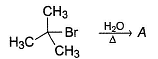
Comprehension Type
Direction (Q. Nos. 23-27) This section contains a paragraph, describing theory, experiments, data, etc.
Five questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
A chemist isolated a compound A with molecular formula C7H13Br. A undergoes very fast SN1 reaction. Spectroscopic evidence indicated that compound A has the following structural characteristics : It contains five sp3-hybridised carbon atoms. Among those five sp3 carbon atoms, three are methyl groups, one CH2 group and one CH group.
It also contains two sp2-hybridised carbon atoms. Also there is only one hydrogen atom attached to sp2 carbons.
The compound contains a total of six aliylic hydrogen atoms.
The carbon atom that holds the Br has one H attached to it.
Working Space
When compound A reacts with boiling water, it undergoes a SN1 reaction and produces two principal products B and C. Both B and C are alcohols with their molecular formula C7H14O. Among the two alcohols, B has the —OH group attached to a sp3 carbon atom that has no H -atoms bonded to it.
Q.
How many stereoisomers are possible for A?
Five questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
It also contains two sp2-hybridised carbon atoms. Also there is only one hydrogen atom attached to sp2 carbons.
The compound contains a total of six aliylic hydrogen atoms.
The carbon atom that holds the Br has one H attached to it.
Working Space
When compound A reacts with boiling water, it undergoes a SN1 reaction and produces two principal products B and C. Both B and C are alcohols with their molecular formula C7H14O. Among the two alcohols, B has the —OH group attached to a sp3 carbon atom that has no H -atoms bonded to it.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
A chemist isolated a compound A with molecular formula C7H13Br. A undergoes very fast SN1 reaction. Spectroscopic evidence indicated that compound A has the following structural characteristics : It contains five sp3-hybridised carbon atoms. Among those five sp3 carbon atoms, three are methyl groups, one CH2 group and one CH group.
It also contains two sp2-hybridised carbon atoms. Also there is only one hydrogen atom attached to sp2 carbons.
The compound contains a total of six aliylic hydrogen atoms.
The carbon atom that holds the Br has one H attached to it.
Working Space
When compound A reacts with boiling water, it undergoes a SN1 reaction and produces two principal products B and C. Both B and C are alcohols with their molecular formula C7H14O. Among the two alcohols, B has the —OH group attached to a sp3 carbon atom that has no H -atoms bonded to it.
Q.
What can be said about the isomerism shown by the two alcohols B and C ?
It also contains two sp2-hybridised carbon atoms. Also there is only one hydrogen atom attached to sp2 carbons.
The compound contains a total of six aliylic hydrogen atoms.
The carbon atom that holds the Br has one H attached to it.
Working Space
When compound A reacts with boiling water, it undergoes a SN1 reaction and produces two principal products B and C. Both B and C are alcohols with their molecular formula C7H14O. Among the two alcohols, B has the —OH group attached to a sp3 carbon atom that has no H -atoms bonded to it.
A chemist isolated a compound A with molecular formula C7H13Br. A undergoes very fast SN1 reaction. Spectroscopic evidence indicated that compound A has the following structural characteristics : It contains five sp3-hybridised carbon atoms. Among those five sp3 carbon atoms, three are methyl groups, one CH2 group and one CH group.
It also contains two sp2-hybridised carbon atoms. Also there is only one hydrogen atom attached to sp2 carbons.
The compound contains a total of six aliylic hydrogen atoms.
The carbon atom that holds the Br has one H attached to it.
Working Space
When compound A reacts with boiling water, it undergoes a SN1 reaction and produces two principal products B and C. Both B and C are alcohols with their molecular formula C7H14O. Among the two alcohols, B has the —OH group attached to a sp3 carbon atom that has no H -atoms bonded to it.
Q.
If the starting compound A is brominated in gas phase in the presence of a Lewis acid catalyst, a tribromide would result from addition of Br2 to . How many different structures of stereoisomers can be drawn for this tribromide?
A chemist isolated a compound A with molecular formula C7H13Br. A undergoes very fast SN1 reaction. Spectroscopic evidence indicated that compound A has the following structural characteristics : It contains five sp3-hybridised carbon atoms. Among those five sp3 carbon atoms, three are methyl groups, one CH2 group and one CH group.
It also contains two sp2-hybridised carbon atoms. Also there is only one hydrogen atom attached to sp2 carbons.
The compound contains a total of six aliylic hydrogen atoms.
The carbon atom that holds the Br has one H attached to it.
Working Space
When compound A reacts with boiling water, it undergoes a SN1 reaction and produces two principal products B and C. Both B and C are alcohols with their molecular formula C7H14O. Among the two alcohols, B has the —OH group attached to a sp3 carbon atom that has no H -atoms bonded to it.
Q.
If the original compound A is treated with LiAIH4 a new compound D(C7H14) would be produced. How many different structure(s) can be drawn for this D ?
A chemist isolated a compound A with molecular formula C7H13Br. A undergoes very fast SN1 reaction. Spectroscopic evidence indicated that compound A has the following structural characteristics : It contains five sp3-hybridised carbon atoms. Among those five sp3 carbon atoms, three are methyl groups, one CH2 group and one CH group.
It also contains two sp2-hybridised carbon atoms. Also there is only one hydrogen atom attached to sp2 carbons.
The compound contains a total of six aliylic hydrogen atoms.
The carbon atom that holds the Br has one H attached to it.
Working Space
When compound A reacts with boiling water, it undergoes a SN1 reaction and produces two principal products B and C. Both B and C are alcohols with their molecular formula C7H14O. Among the two alcohols, B has the —OH group attached to a sp3 carbon atom that has no H -atoms bonded to it.
Q.
If the starting compound A is treated with ethanolic solution of KOH in boiling condition, a further new compound E (C7H12) is produced as major product by E2 elimination reaction. Which of the following statement will be consistent with E?
A correct statement about transition state of SN2 reaction is
What is the correct increasing order of reactivity of the followings in SN2 reaction ?
I. CH2 = CHCH2 — Br
II. CH2 = CH— I
III. CH3CH2CH2 — I
IV. CH3OCH2CH2 — I
What is the correct increasing order of reactivity of the following in the SN2 reaction?
Consider the two lines shown in the diagram given below.
Q.
In a SN2 reaction, these two lines compare the effect of the

















