Test: Reactions of Alcohols, Phenols(27 Nov) - JEE MCQ
10 Questions MCQ Test - Test: Reactions of Alcohols, Phenols(27 Nov)
Only One Option Correct Type
Direction (Q. Nos. 1-8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
The correct order of acidic strength of the following alcohols is
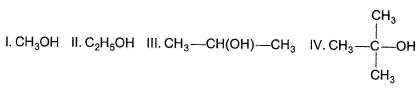
Which compound given below has the highest solubility in water ?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
What is the correct increasing order of acidity of the following?
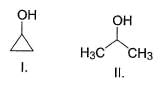
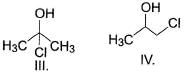


What is the order of solubility of the following in water?
Which of the following can be used for the distinction of ethanol from phenol?
Consider the following reaction,
The above reaction can best be brought about by
Consider the following reaction,
The major product is
One or More than One Options Correct Type
Direction (Q. Nos. 9-12) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Consider the compound shown below,
Select the correct statement(s).
Methanol and ethanol can be distinguished by
Consider the following substituted ethanol G—CH2CH2OH
Which group(s) when present as G gives greater equilibrium of gauche conformer than its anti counterpart ?


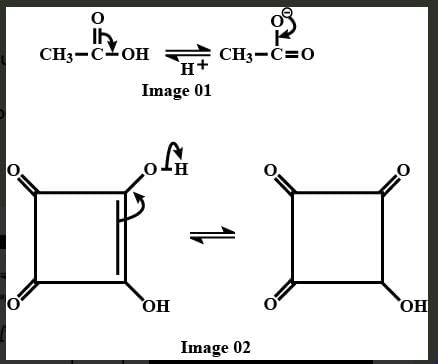 It is more acidic compound bc2 this molecule loss H+ ions very easily. as compared to compound [A]
It is more acidic compound bc2 this molecule loss H+ ions very easily. as compared to compound [A]














