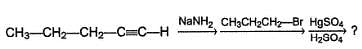Test: Preparation of Aldehydes and Ketones(7 Dec) - JEE MCQ
10 Questions MCQ Test - Test: Preparation of Aldehydes and Ketones(7 Dec)
Only One Option Correct Type
Direction (Q. Nos. 1-12) This section contains 12 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
An optically active organic compound has molecular formula C5H12O(X). X on oxidation with CrO3/H2SO4 gives an achiral C5H10O. Hence, X could be
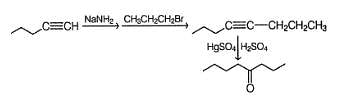
Which of the following reaction will not produce an aldehyde?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which reagent below cannot reduce an acid chloride to an aldehyde?
The incorrect statement regarding oxo process for synthesis of an aldehyde is
All of the following reaction gives atleast one ketone as a significant organic product except
All of the following results in the formation of an aldehyde except
Consider the following reaction,
All of the following reagents can bring about the above transformation except
A hydrocarbon X(C7H12) on ozonolysis followed by the treatment with (CH3)2S gives C7H12O2 which gives positive Tollen’s test as well as positive iodoform test. The compoppd below satisfying the criteria of X is
A hydrocarbon X has molecular formula C5H10 X on treatment with B2H6 in H2O2 /NaOH gives an optically active C5H12O which on treatment with CrO3/HCI / pyridine gives C5H10O which is still chiral. Which of the following can be a product of reductive ozonolysis of X?
What is the final major product of the following reaction