Test: Spectroscopy Level - 3 - Chemistry MCQ
30 Questions MCQ Test - Test: Spectroscopy Level - 3
The spectroscopic data for an organic compound with molecular formula C10H12O2 are given below. IR band around 1750 cm–1. 1H NMR 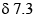 (m, 5H), 5.85 (q, 1H, J = 7.2 Hz), 2.05 (s, 3H), 1.5 (d, 3H, J = 7.2 Hz) ppm. The compound is:
(m, 5H), 5.85 (q, 1H, J = 7.2 Hz), 2.05 (s, 3H), 1.5 (d, 3H, J = 7.2 Hz) ppm. The compound is:
The correct order of the 1H NMR chemical shift values 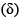 for the indicated hydrogens (in bold) in the following compounds is:
for the indicated hydrogens (in bold) in the following compounds is:
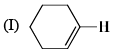
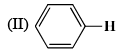

| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the following compound show only two signals in 1H NMR and a strong IR bond at ~ 1690 cm-1:
The correct order of 1H NMR chemical shift values for the labeled methyl groups in the following compound is:
The 1H NMR spectrum of a compound with molecular formula C3H7NO shows the following features:
Which of the following is in agreement with this information:
The two fine–structure components of a nuclear magnet ic resonance transit ion are observed at chemical shifts of 2.142 and 2.208 ppm in a 300 MHz NMR spectrometer. Calculate the coupling constant:
A C5H12O2 compound has strong infrared absorption at 3300 to 3400 cm-1 The 1H NMR spectrum has three singlets at δ 0.9 , δ 3.45 and δ3.2 ppm; relative areas 3:2:1. The 13C NMR spectrum shows three signals all at higher field than δ100 ppm. Suggest a structure for this compound.
The 1H NMR spectrum of a compound A shows a doublet and a septet. Which one of the following statements is true:
A compound of formula C5H12 gives one signal in the 1H NMR and two signals in the 13C NMR spectra. The compound is:
The most appropriate spectroscopy for the identification of a nitrile group is:
Compound I gives a strong infrared absorption at 1730 cm-1. 1H NMR spectrum indicates that it has two types of hydrogen atoms; one H atom appearing as signlet at = 9.7 ppm and 9H atoms appearing as a singlet at
= 1.2 ppm. The structure of I is:
The functionality that shows a typical characteristic peak at 2250 cm-1 in IR is:
Consider a 1H NMR spectrum in which a quartet and a doublet appeared at 9.72 and 2.40 ppm, respectively. Which of the following compounds is the most probable one:
The order of decreasing chemicals shift in 1H NMR for the underlined hydrogens is:
(I) H3C – CH2 – CH3
(II) H3C – O – CH2 – CH3
(III) Cl2 – CH – O – CH2 – CH3
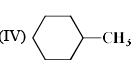
(IV) Cl – CH2 – O – CH2 – CH3
The number of normal modes of vibration in the benzene molecule is:
An organic compound (MF: C8H10O) exhibited the following 1H NMR spectral data: 2.5 (3 H,s), 3.8 (3H, s), 6.8 (2 H, d, J 8 Hz), 7.2 (2 H, d, J 8 Hz) ppm. The compound among the choices is:
The 1H NMR spectrum of 1, 4-dimethoxybenzene will have:
Appropriate 1H NMR chemical shifts for the protons A-D for the following compound are:
Compound A and B exhibit two singlets, each in their 1H NMR spectra. The expected chemical shifts are at :
In the 1H NMR spectrum recorded at 293 K, an organic compound (C3H7NO), exhibited signals at δ 7.8 (1H, s), 2.8 (3H, s) and 2.6 (3H, s). The compound is:
An organic compound exhibited the following 1H NMR spectra data:
7.80 (2 H, d, J = 8 Hz), 6.80 (2 H, d, J = 8 Hz), 4.10 (2 H, q, J = 7.2 Hz), 2.4 (3H, s), 1.25 (3 H, t, J = 7.2 Hz)
The compound, among the choices given below is,
In NMR spectroscopy the product the nuclear ‘g’ factor (gN), the nuclear magneton (βN) and the magnetic field strength (B0) gives the:
Among the isomers of C4H6 given below, the compound which exhibits an absorption band at 3300 cm-1 in the IR spectrum, is:
Which of the following absorptions is shown by 1, 3-butadiene in its UV absorption spectrum recorded in n-hexane (εmax is the molar absorptivity):
Match the observed principal absorptions in the visible spectrum shown in List-I wit h the bond shows this absorption in List–II:
The correct structure of the compound based on the following characteristic spectral data is IR: 1736 cm–1
1H NMR: 3.59 (s, 3H), 3.32 (t, 2H), 2.25 (t, 2H), 1.85-1.75 (m, 2H), 1.73-1.62 (m, 2H)
13C NMR: 174.0, 51.0M 32.9, 32.8, 31.0, 23.0
In atomic absorption spectroscopy, the atomization process utilizes:
The absorption at λmax 279 nm in the UV spectrum of acetone is due to:
An organic compound having the molecular formula C10H14 exhibited two singlets in the 1H NMR spectrum and three signals in the 13C NMR spectrum. The compound is:
A compound with molecular formula C5H10O, exhibit following 1H NMR spectral data - 0.95 (6H, d),
2.10 (3H, S),
2.43 (1H, m). The structure is:


















