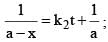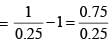Chemistry - 2013 Past Year Paper - IIT JAM MCQ
30 Questions MCQ Test - Chemistry - 2013 Past Year Paper
Which one of the following order of the carbonates is Correct for their decomposition temperature?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The Correct order of CO vibrational stretching frequency in the following complexes is
(I) (PF3)3Mo(CO)3
(II) (PCl3)3Mo(CO)3
(III) {P(OMe)3}3Mo(CO)3
(I) (PF3)3Mo(CO)3
(II) (PCl3)3Mo(CO)3
(III) {P(OMe)3}3Mo(CO)3
Among the following, the ligand that BEST stabilizes low oxidation state of tungsten (W) is
The function y = x exp (–x2) has a minimum at x = The second derivative of the function at
the minimum is
For a particular reaction at constant temperature, a plot of inverse of reactant concentration versus time is a straight line with a slope of 4.0 × 10–2 L mol–1 s–1. The time required (in seconds) for 1.0 M of reactant to decrease to 0.25 M is:
For a physisorption process, which one of the following statements is NOT correct?
The Correct order of stability of the following carbonium ions is
Which one of the following statements is Correct?
The reaction of anhydrous FeCl2 with sodium-pentadienyl in ether gives an air-stable diamagnetic orange solid, which on oxidation gives an air-sensitive paramgnetic blue-green compound in solution.
The blue-green compound is _____________
CaO, VO and MnO have octahedral coordination of the metal ions in a rock-salt structure. The correct increasing order of their lattice enthalpies is
The structure of sulphur dioxide molecule (SO2) may be given as:
The vapour pressures of solid and liquid chlorine are given by
where Psolid and Pliq are the vapour pressures (in Torr) of solid and liquid chlorine near the triple point, respectively and T is the absolute temperature. The ratio of the slope of the solid-gas curve to the slope of the liquid gas curve at the triple point in the P-T diagram is________
For unnormalized wave-function , the number of radial node(s) is __________
A hypothetical element (atomic weight = 300) crystallizes in a simple cubic lattice. For this crystal, the first order X-ray diffraction with wavelength of 5 Å appears at an angle of 30º. The density of the crystal is _____________g cm–3. [Avogadro number, NA = 6.02 × 1023]
MnO4– (aq) + Zn(s) + H3O+ (aq) → Mn2+ (aq) + Zn2+ (aq) + H2O (ℓ) For the above reaction if the equilibrium constant at 298 K is represented by 10x, then the value of X is _________
[Given: The standard cell potential Eº = 2.4 V and = 0.06 V at 298 K]
The rotational energy barrier between the most stable and the least stable conformations of 2, 3-dimethylbutane along C2–C3 bond is ___________kcal mol–1. [Given: The energies (kcal mol–1) for H/CH3 eclipsing = 1.8, CH3/CH3 eclipsing = 2.9 and CH3/ CH3 gauche = 0.9]
The number of peaks or signals in 1H NMR of N, N-dimethylformamide (DMF) at 25ºC is ______
Calixene is a polar hydrocarbon with a high dipole moment. The most stable dipolar canonical structure is _________
A mixture of C3H8 and oxygen in 1L closed vessel has an internal pressure of 4 atm at 100ºC. When the mixture is ignited, the reaction produces CO2(g) and H2O(g) until all oxygen is consumed.
After the reaction, pressure of the vessel is 4.2 atm at the same temperature. Calculate the weight of oxygen present before the reaction. [Gas constant, R = 0.082 L atm mol–1 K–1].
The following reaction is carried out at 1 atm and 300 K 2H2(g) + O2(g) → 2H2O (l) ΔU
for the above reaction is 550 kJ. Assuming ideal gas behaviour for H2 and O2, calculate the value of ΔH. The value of gas constant, R = 0.082 L atm mol-1 K-1 = 8.314 mol-1 K-1.
[Given: The volume of 1 mole of liquid water is 18 mL under the above reaction condition]
At 298 K, calculate the solubility of metal sulfide, MS(s), in a saturated solution of H2S where the concentration of H2S and pH are maintained at 0.1 M and 3.0, respectively Given at 298 K,
K = 10–7
K = 5 × 10–19
For each of the following metallo-proteins identify the metal-ion at the active-site and the function of the proteins: deoxy-hemoglobin, deoxy-myoglobin, oxy-hemocyanin, cytochrome-C and carbonic anhydrase.
A solution containing 250 ppm of CuSO4 · 5H2O (formula weight = 250) has an absorbance of 0.1 measured in 1 cm cell at 600 nm. Calculate the molar absorptivity (e) of CuSO4 · 5H2O in L M-1 cm-1 . When 25 mL of Na2EDTA (aq) solution is titrated against Na2EDTA (aq) solution, it consumes at 50 mL of Na2EDTA (aq) solution. Calculate the concentration of Na2EDTA (aq) solution in moles L-1 .
Assume the complex [NI(PPh3)2(SCN)2] is paramagnetic. The analogous complex of Pd(II) is diamagnetic. Draw all the probable isomers for both the complexes considering SCN– is an ambidentate ligand.
Write the structure of A to E in the following reaction sequence:
Write the structures of F to J in the following reaction scheme:
[DiBAL-H = diisobutylaluminium hydride]
Propose a mechanism for the following reaction. Show stepwise correct reactive intermediates
Complete the following reaction sequence and write structures of K to O.


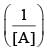 vs ‘t’ graph gives straight line for a second order reaction
vs ‘t’ graph gives straight line for a second order reaction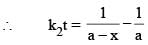 ... (1)
... (1)