IIT JEE Main Mock Test 5 - JEE MCQ
30 Questions MCQ Test - IIT JEE Main Mock Test 5
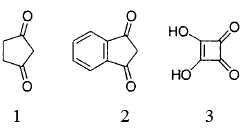
The correct order of acidic strength of the compounds, 1 – 3 is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The correct order of bond angle of the following compound is
The rate law for one of the mechanism of the pyrolysis of CH3CHO at 520 oC and 0.2 bar is
The overall activation energy Ea in terms of rate law is:
The stopping potential (V0) of photosensitive surface varies with the frequency of incident radiation as shown in the following figure. The work function of photosensitive surface.
5.6 litre of an unknown gas at NTP requires 12.5 calorie to raise its temperature by 10 oC at constant volume.
The value of ( PVm )P→0 of a real gas is independent of the nature of gas because
Which of the following oxide cannot be reduced by carbon reduction?
Which of the following can show both optical and geometrical isomeris?
A reversible process is performed in such a way that the system passes from state 1 to state 2 by path-I and then from state 2 to state one by path
II as shown in figure 5.
For the above process
That the quantity is not depends on the path of the reaction, but depends on initial and final state of the system.
The quantity in the above process is called
A reversible process is performed in such a way that the system passes from state 1 to state 2 by path-I and then from state 2 to state one by path
II as shown in figure 5.
For the above process
That the quantity is not depends on the path of the reaction, but depends on initial and final state of the system.
In thermodynamics, a process is called reversible when:
Observe the given curve for a substance and identify the correct statement.
There are some deposits of nitrates and phosphates in the earth’s crust. Nitrates are more soluble in water. Nitrates are difficult to reduce under the laboratory conditions but microbes do it easily. Ammonia forms large number of complexes with transition metal ions.
Select the correct statement among the following:
There are some deposits of nitrates and phosphates in the earth’s crust. Nitrates are more soluble in water. Nitrates are difficult to reduce under the laboratory conditions but microbes do it easily. Ammonia forms large number of complexes with transition metal ions.
Nitrates are mainly produced for use as fertilizers in agriculture because of their
The degree of unsaturation in the product of following reaction is
Which chlorine in the following compound is most reactive towards nucleophilic (weak base) substitution reaction?
In how many of the following compound plane of symmetry is present.
CH3Cl, CH2Cl2, XeF4, CH3CH=C=CHCH3, CH3CH(OH)Cl
How many corners of tetrahedral SiO44− unite shared in cyclic silicates?
A solution is prepared by mixing equal volume of two solutions A and B with pH 8 and 10 respectively. The pH of resultant solution is given as pH = x + 1 – log 2. The value of x is
P4O6 contains N lone pairs of electron, M number of P-P bonds and X number of P – O bonds. The value of N - M + X is
How man y π-e lectrons are present in the final product in the following reaction?
When the hematite ore is burnt in air with coke in blast furnace, The change in oxidation number of carbon in the reduction zone of the furnace is
How many lone pair of electrons at Xe atom are present in XeOF4?
Range of the function, (where sgn (⋅) denotes the signum function and [⋅] greatest integer function, {.} is a fractional function)
Let the circles S1 ≡ x2 + y2 – 4x – 8y + 4 = 0 and S2 be its image in the line y = x, the equation of the circle touching y = x at (1, 1) and orthogonal to S2 is

















