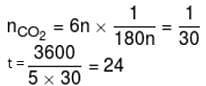JEE Main Chemistry Test- 5 - JEE MCQ
25 Questions MCQ Test - JEE Main Chemistry Test- 5
Crotonaldehyde (CH3CH = CHCHO) can be easily oxidised to crotonic acid (CH3CH = CHCOOH) using
Which of the following aldehydes forms a stable hydrate ?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
When propyne is treated with aqueous H2SO4 in the presence of HgSO4 , the major product obtained is
An organic compound, C3H6O does not give a precipitate with 2, 4-dinitrophenylhydrazine reagent and does not react with metallic sodium. It could be
Identify the compounds A and B in the following reaction sequence.
An organic compound ‘A’ has the molecular formula C3H6O It undergoes iodoform test. when saturated with HCl, it gives ‘B’of molecular formula C9H14O ‘A’ and ‘B’ respectively are
With which of the following reagents, carbonyl compound shows addition cum elimination reaction ?
At a certain temperature, the half-life periods for the catalytic decomposition of NH3 were found to be as follows :-
Pressue (mm Hg) 50 100 200
Half-life period (hrs.) 3.52 1.76 0.88
What will be the pressure when the half-life period is 2.5 hours.
How many of the following elements form amphoteric oxides?
Si, P, N, B, Sn, Pb, O
One gm of activated carbon has surface area of 1000 m2. Considering complete coverage as well as mono molecular adsorption, how much ammonia at 1 atm and 273 K would be adsorbed on the surface of 44/7 g carbon if radius of ammonia molecule is 10–8 cm (NA = 6 × 1023)
1gm of dry green algae absorbs 5 moles of CO2 per hour by photo synthesis. If fixed carbon atoms were all stored in the form of starch (C6H12O6)n after photo synthesis then calculate time required ( in sec.) to double the weight of algae.
In the electronic configuration of Zn (Z = 30) find the total number of occupied orbitals which do not possess any nodal plane :-