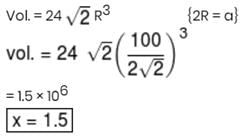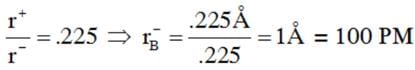JEE Main Chemistry Test- 8 - JEE MCQ
Test Description
25 Questions MCQ Test - JEE Main Chemistry Test- 8
JEE Main Chemistry Test- 8 for JEE 2024 is part of JEE preparation. The JEE Main Chemistry Test- 8 questions and answers have been prepared
according to the JEE exam syllabus.The JEE Main Chemistry Test- 8 MCQs are made for JEE 2024 Exam.
Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for JEE Main Chemistry Test- 8 below.
Solutions of JEE Main Chemistry Test- 8 questions in English are available as part of our course for JEE & JEE Main Chemistry Test- 8 solutions in
Hindi for JEE course.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free. Attempt JEE Main Chemistry Test- 8 | 25 questions in 60 minutes | Mock test for JEE preparation | Free important questions MCQ to study for JEE Exam | Download free PDF with solutions
JEE Main Chemistry Test- 8 - Question 1
The molal depression constant for water is 1.86. The depression constant for 100g 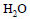 is
is
Detailed Solution for JEE Main Chemistry Test- 8 - Question 1
JEE Main Chemistry Test- 8 - Question 2
Urea is added to 2 litre of water to such an extent that 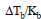 becomes equal to 1/100. The weight of urea added is
becomes equal to 1/100. The weight of urea added is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
JEE Main Chemistry Test- 8 - Question 3
The moleucular weight of NaCl (degree of dissociation=x) determined by the osmotic pressure method, is found to be different from its actual molecular wieght (M).Which of the following relationship is correct ?
JEE Main Chemistry Test- 8 - Question 4
The osmotic pressure of glucose solution (400mm),on dilution, decreased to 100 mm. The extent of dilution is
JEE Main Chemistry Test- 8 - Question 5
At what temperature does an aqueous solution containing 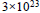 molecules of a noneelectrolyte substance in 250g of water freeze ?
molecules of a noneelectrolyte substance in 250g of water freeze ? 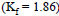
JEE Main Chemistry Test- 8 - Question 6
The vapour pressure of a solvent decreased by 10mmHg when a nonvolatile solute was added to the solvent. The mole fraction of the solute in the solution
is 0.2. What would be the mole fraction of the solvent if the decrease in the vapour pressure is to be 20 mmHg
is 0.2. What would be the mole fraction of the solvent if the decrease in the vapour pressure is to be 20 mmHg
Detailed Solution for JEE Main Chemistry Test- 8 - Question 6
JEE Main Chemistry Test- 8 - Question 8
A metal crystallizes with a face-centered cubic lattice. The edge of the unit cell is 408 pm. The diameter of the metal atom is
Detailed Solution for JEE Main Chemistry Test- 8 - Question 8
JEE Main Chemistry Test- 8 - Question 9
Alkene R–CH–CH = 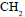 reacts with
reacts with 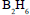 in the presence of
in the presence of 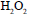 to give
to give
JEE Main Chemistry Test- 8 - Question 10
The order of reactivity of the following alcohols towards conc. HCl is
i.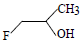 ii.
ii. 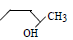
iii.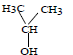 iv.
iv. 
i.
iii.
Detailed Solution for JEE Main Chemistry Test- 8 - Question 11
JEE Main Chemistry Test- 8 - Question 12
Increasing order of acid strength among tertiary butanol,isopropanol and ehanol is
JEE Main Chemistry Test- 8 - Question 17
Propene is allowed to react with HI. The product (A) is then treated with 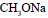
to give a new product (B)
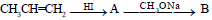
The final product (B) is
to give a new product (B)
The final product (B) is
JEE Main Chemistry Test- 8 - Question 19
The utility of the polymers in various fields is due to their mechanical properties like tensile strength,elasticity, toughness etc.These properties mainly depend upon intermolecular forces like van der Waal’s forces and hydrogen bonding operating in polymer molecules. Polymers have been classified on this
basis,e.g.(1) Elastomers (2) Thermoplacstics (4) Thermosetting. Hence
The molecular forces of attraction are weakest in
basis,e.g.(1) Elastomers (2) Thermoplacstics (4) Thermosetting. Hence
The molecular forces of attraction are weakest in
JEE Main Chemistry Test- 8 - Question 20
The utility of the polymers in various fields is due to their mechanical properties like tensile strength,elasticity, toughness etc.These properties mainly depend upon intermolecular forces like van der Waal’s forces and hydrogen bonding operating in polymer molecules. Polymers have been classified on this
basis,e.g.(1) Elastomers (2) Thermoplacstics (4)Thermosetting. Hence
Which of the following have usually a linear structure
basis,e.g.(1) Elastomers (2) Thermoplacstics (4)Thermosetting. Hence
Which of the following have usually a linear structure
*Answer can only contain numeric values
JEE Main Chemistry Test- 8 - Question 21
Given length of side of HCP is 100/√2 pm. The volume of HCP unit cell is (in pm3) x × 106 then x will be–
Detailed Solution for JEE Main Chemistry Test- 8 - Question 21
*Answer can only contain numeric values
JEE Main Chemistry Test- 8 - Question 22
The radius of A+ in ionic compound AB is .225Å If AB has ZnS structure, the ideal radius of B– will be (in PM)
Detailed Solution for JEE Main Chemistry Test- 8 - Question 22
*Answer can only contain numeric values
JEE Main Chemistry Test- 8 - Question 23
K[Co(CO)4], K3[Co(CN)6] Find the difference between oxidation number of central metal in the given complexes.
*Answer can only contain numeric values
*Answer can only contain numeric values
JEE Main Chemistry Test- 8 - Question 25
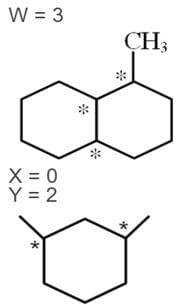
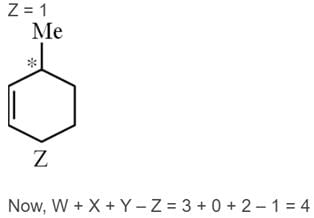
Number of chiral carbon atoms in the given compounds are W, X, Y and Z respectively then Calculate the I, II, III & IV value of W + X + Y– Z.
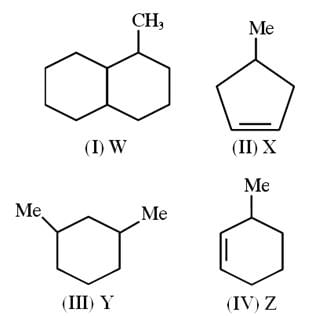
Detailed Solution for JEE Main Chemistry Test- 8 - Question 25
Information about JEE Main Chemistry Test- 8 Page
In this test you can find the Exam questions for JEE Main Chemistry Test- 8 solved & explained in the simplest way possible.
Besides giving Questions and answers for JEE Main Chemistry Test- 8, EduRev gives you an ample number of Online tests for practice
Download as PDF