(18-01-2018) Major Test - 1 - IIT JAM MCQ
30 Questions MCQ Test - (18-01-2018) Major Test - 1
Correct order of bond length of p, q, r, x and y given in following compound is:
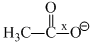
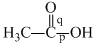
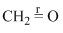
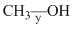
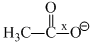
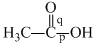
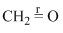
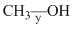
Which of the following reaction profile represent the following energy diagram:


| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
For (CH3)2S correct sequence of Lewis acidic strength:
According to VSEPR theory, the molecule/ion having ideal tetrahedral shape is:
The pH of the blood is maintained by the balance between H2CO3 and NaHCO3. If the amount of CO2 in the blood is increased how will it affect the pH of blood?
The change in the hybrid state of BeCl2 in the solid state from vapour state is:
A mixture of two amino acids having pH 9.60 and 5.40 can be separated:
An aqueous solution of hydrated aluminium chloride contains the following set of ions:
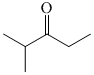
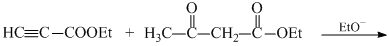
Which sequence of steps describes the best synthesis of 2-methyl-3-pentanone?
The angular part of the wave function for the electron in a hydrogen atom is proportional to 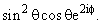 . The values of the azimuthal quantum number (l) and the magnetic quantum number (m) are, respectively
. The values of the azimuthal quantum number (l) and the magnetic quantum number (m) are, respectively
What can be the maximum pressure to be given to a plastic for blow molding process?
A molecule having formula PQ2H8 has the following structural features (H is hydrogen atom):
(I) P and Q both belong to period 2 of periodic table.
(II) P and Q have two and three balance electrons respectively.
(III) There are four (2c—2e–) bonds and four (3c—2e–) bonds in the molecule.
(IV) In P and Q both form atleast one (2c—2e–) bond.
Based on above structural information which one of the following structures is most appropriate for PQ2H8?
In the thermite process, iron oxide is reduced to molten iron by aluminium powder because:
A solution containing a group-IV cation gives precipitate on passing, H2S. A solution of this precipitate in dil. HCl produces a white precipitate with NaOH solution and bluish-white precipitate with basic potassium ferrocyanide. The cation is:
A metal ‘M’ reacts with nitrogen gas to afford ‘M3N’. ‘M3N’ on heating at high temperature gives back ‘M’ and on reaction with water produces a gas ‘B’. Gas ‘B’ reacts with aqueous solution of CuSO4 to form a deep blue compound. ‘M’ and ‘B’ respectively are:
The major product formed in the following reaction is:

The n factor of K4[Fe(CN)6] in the given reaction would be:
Zn2++ K4[Fe(CN)6]→ K2Zn3[Fe(CN)6]2+ KΘ
For a given nuclear fission reaction of 235U
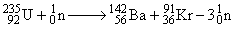
The amount of energy (in kJ/mol) released during this process is (given 235U = 235.0439 amu,
142Ba = 141.9164 amu, 91Kr = 90.9234 amu, neutron = 1.00866 amu):
An organic compound A(C8H16O2) on treatment with an excess of methylmagnesium chloride generated two alcohols B and C, whereas reaction of A with lithium aluminium hydride generated only a single alcohol C. Compound B on treatment with an acid yielded an olefin ?(C6H12), which exhibited only a singlet at ppm in the 1H NMR spectrum. The compound A is:
The major product formed in the following reaction is:
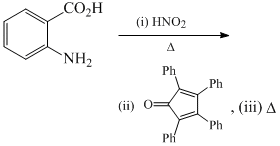
The major product formed in the following reaction is:
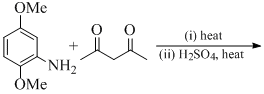
The species with highest magnetic moment (spin only value) is:
The molal freezing point depression constant of benzene (C6H6) is 4.90 K kg mol–1. Selenium exists as a polymer of the type Sex. When 3.26 g of selenium is dissolved in 0.112 °C lower than pure benzene. The correct molecular formula of selenium is:
Equivalent conductance of BaCl2, H2SO4 and HCl are x1, x2 and x3 S cm2 equiv–1 at infinite dilution. If specific conductance of saturated BaSO4 solution is y S cm–1 then Ksp of BaSO4 is:



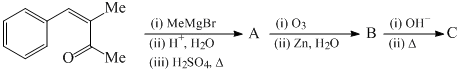
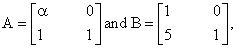 then the value of a for which A2 = B is:
then the value of a for which A2 = B is: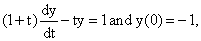 then y(1) is equal to:
then y(1) is equal to: