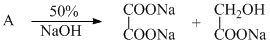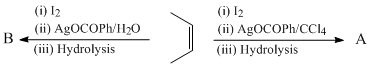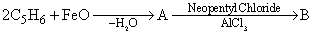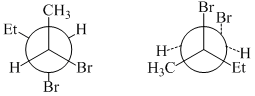(29-01-2018) Major Test - 4 - IIT JAM MCQ
30 Questions MCQ Test - (29-01-2018) Major Test - 4
Which of the following carbonates of alkali metals has least thermal stability?
Fluorescence life time of a molecule is 10 ns. If fluorescence quantum yield is 0.1, what is rate constant of fluorescence decay?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Aluminium-25 decay by emitting a positron. The species immediately produced has:
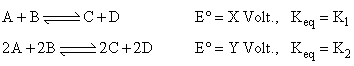
For a cell reaction, consider the following equations:
Which of the following amino acid is most hydrophobic?
Arrange the following in decreasing order of 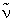 (cm–1) stretching frequency.
(cm–1) stretching frequency.
(I) Mo(CO)3(NMe3)3 (II) Mo(CO)3[P(OPh)3]3
(III) Mo(CO)3(PMe3)3 (IV) Mo(CO)3(PCl3)3
emf of Cd-cell is 1.018 V at 25°C. The temperature coefficient of cell is –5.2 × 10–5 VK–1. How cell temperature will change during operation?
In the extraction of copper from its sulphide ore, metal is finally obtained by the oxidation of cuprous sulphide with :
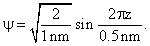 Calculate En if ψ represents state of a particle in 1-D box of length 1nm.
Calculate En if ψ represents state of a particle in 1-D box of length 1nm.
t1/2 (half-life period) of 1st order reaction is 10 min. Starting with 10 mol L–1, rate after 20 min is:
25 mL of hydrogen and 18 mL of Iodine when heated in closed container, produced 30.8 mL of HI at equilibrium. Calculate degree of dissociation of HI at same temperature:
For a surface inactive substance 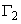 (excessive concentration of solute per unit area of surface) is essentially:
(excessive concentration of solute per unit area of surface) is essentially:
Which of the following does not show facial & meridonial isomerism
A. Kw of water at 100°C is 55 times than that at 25°C
B. A Solution at 100°C has pH = 5.
Choose the correct among following pairs if statement 2 is in reference to statement 1:
Consider μ1, μ2, μ3 are dipole moments of pyrrole, furan, thiopene respectively. Which of the following holds true:
For water, if pressure is increased from 4.6 mm Hg to 400 mm Hg, then, change in temperature should be:
Which of the following is true about osazone formation in carbohydrates:
If bond length of CO bond in carbon monoxide is 1.128 Å, then what is the value of CO bond length in Fe(CO)5?
Consider borax. Chemical formula of which is Na2B4O7. 10H2O. Choose the incorrect statement:
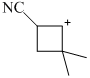
Which of the following will give different product on hydrolysis (among others)?
1H NMR spectrum of a compound with molecular formula C4H9NO2 shows:
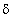 5.30 (broad, 1H);
5.30 (broad, 1H); 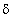 4.10 (q, 2H);
4.10 (q, 2H); 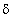 2.80 (d, 3H);
2.80 (d, 3H); 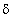 1.20 (t, 3H). The structure of compound that is consistence with the above data is:
1.20 (t, 3H). The structure of compound that is consistence with the above data is:
sin2θ = 0.1198, 0.2395, 0.3588, 0.493, 0.5984. Find lattice type:


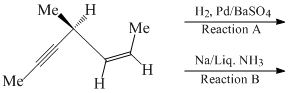
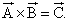 Then which of the following holds true?
Then which of the following holds true?