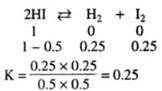BITSAT Chemistry Test - 4 - JEE MCQ
30 Questions MCQ Test - BITSAT Chemistry Test - 4
Which of the following compound is resistant to nucleophilic attack by hydroxyl ions?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
100 mL of PH₃ on decomposition produced phosphorus and hydrogen. The change in volume is
Which of the following sets of quantum numbers belongs to highest energy?
For principle quatum number n = 4, the total number of orbitals having l = 3 is
The reaction of calcium with water is represented by the equation Ca + 2H₂O → Ca(OH)₂ + H₂ what volume of H₂, at STP would be liberated when 8 g of calcium completely reacts with water
The heat required to raise the temperature of a body by 1K is called
For reaction aA→xP, when[A]=2.2mM, the rate was found to be 2.4mM s⁻1. On reducing concentration of A to half, the rate changes to 0.6mM s⁻1. The order of reaction with respect to A is
Which of the following is isoelectronic with carbon atom?
The soaps are salts of higher fatty acids containing chains of
A heat engine absorbs heat Q1 at temperature T1 and heat Q2 at temperature T2. Work done by the engine is (Q1 + Q2). This data
At certain temperature, 50% of HI is dissociated into H2 and I2, the equilibrium constant is
When H₂O₂ is added to acidified solution of dichromate ion, then deep blue colour is produced due to the formation of
Which of the following series involves gradual filling of 5f-level?
Consider the following four electrodes
A = Cu+2 0.0001 M | C u s
B = Cu+2 0.1 M | C u s
C = Cu+2 0.01 M | C u s
D = Cu+2 0.001 M | C u s
If the standard reduction potential of Cu+2 ∕ C u is + 0.34 V , the reduction potential (in volts) of the above electrodes follow the order
Which of the following compounds can exhibit functional isomerism?
Which of the following alkyl halides is used as a methylating agent?
A metal X on heating in nitrogen gas gives Y, Y on treatment with H₂O gives a colourless gas which when passed through CuSO₄ soution gives a blue colour. Y is