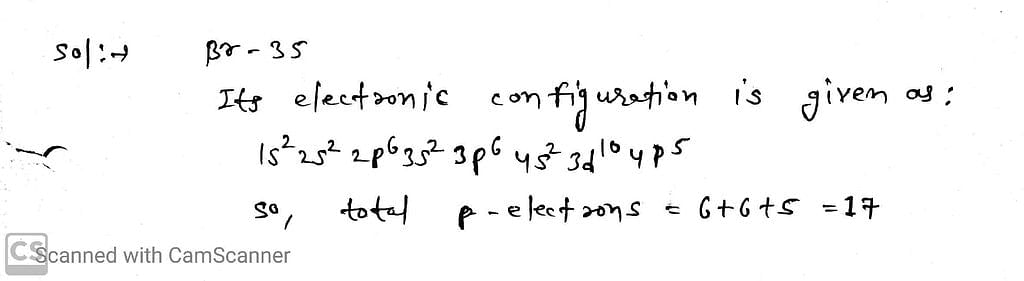BITSAT Chemistry Test - 1 - JEE MCQ
30 Questions MCQ Test - BITSAT Chemistry Test - 1
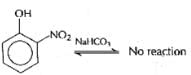
Which of the following will not be soluble in sodium carbonate solution?
Which of the following expressions gives the de-Broglie relationship?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
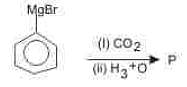
Which of the following reactions is used for detecting presence of carbonyl group ?
The total number of electrons present in all the ρ-orbitals of bromine is
A compound possesses 8% sulphur by mass. The least molecular mass is
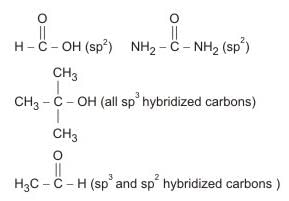
The compound with carbon uses only its sp3 hybrid orbitals for bond formation is
Which of the following gives maximum energy in metabolic process?
Among the following the bond with highest bond dissociation energy is
The enthalpy change (∆H) for neutralisation of 1 M HCl by caustic soda in dilute solutions at 298 K is
A and B are gaseous substances which react reversibly to give two gaseous substances C and D,accompanied by the liberation of heat.When the reaction reaches equilibrium,it is observed that Kp = Kc.The equilibrium cannot be disturbed by
The rate constant of first order reaction whose half life is 480 sec is
Which of the following elements has the lowest ionisation potential
A system is said to be in thermodynamic equilibrium when it is in
The detergents which are most suitable for cleansing synthetic fibres, are
Four successive members of the first row transition elements are listed below with their atomic no. Which of them is expected to have the highest third ionisation enthalpy?
Low spin complexes are generally formed by the elements of
Which of the following isomers will have the highest boiling point ?
When n-propyl iodide is heated with alcoholic KOH,one of the products is
The process of separation of racemic mixture into its constitutes is called
The solubility of AgI in NaI solution is less than that in pure water because
How many monochlorobutanes will be obtained on chlorination of n-butane?
Of the following which is paramagnetic and has three electron bond in its structure