JEE Main Chemistry Mock Test- 9 - JEE MCQ
25 Questions MCQ Test - JEE Main Chemistry Mock Test- 9
The reaction of acetaldehyde with HCN followed by hydrolysis gives a product which exhibits
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which one of the following reactions occur at the cathode?
When benzenediazonium chloride in hydrochloric acid reacts with cuprous chloride, then chlorobenzene is formed. The reaction is called
The Δ H value for the reaction H2 + Cl2 → 2 HCl is − 44.12 K.cal. If E1 is the activation energy of the reactants and E2 is the activation energy of the products, then for the above reaction
How many unit cells are present in a cube-shaped ideal crystal of NaCl of mass 1.0 g?(Atomic weights of Na = 23 and Cl = 35.5)
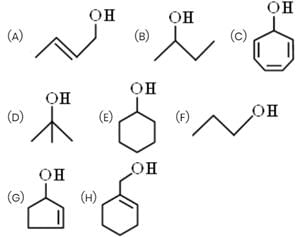
How many alcohols give immediate turbidity on heating with Lucas Reagent?
If total number of chiral centre in α-D-Glucopyranose is 'P' and sucrose is 'Q' then what would be value of (P × Q)?
At 25° C, the solubility product of Mg(OH)2 is 1.0 × 10–11. At which pH, will Mg2+ ions start precipitating in the form of Mg(OH)2 from a solution of 0.001 M Mg2+ ions ?
Successive ionisation potential of an element are 7.6 ev, 12.9 ev, 27 ev, and 92.5 ev, then find number of electrons in the valence shell of the element.
What volume in ml of 5 M H2SO4 should be added to 150 ml of 1 M H2SO4 to obtain a solution of molarity = 2.5 M, if upon mixing volume of solution decreases by 10% ?


















