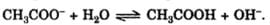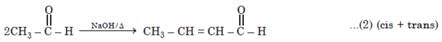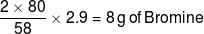JEE Main Chemistry Mock Test- 1 - JEE MCQ
25 Questions MCQ Test - JEE Main Chemistry Mock Test- 1
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Chemical equilibrium is dynamic in nature, because
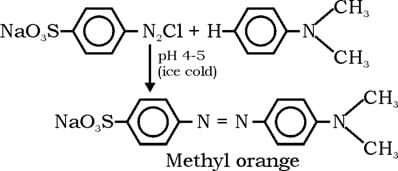
The indicator that is obtained by coupling the diazonium salt of sulphanilic acid with N,N-dimethylaniline is
The standard e.m.f. of a galvanic cell, with standard electrode potentials of zinc equal to -0.76 V and that of copper equal to + 0.34 V, is
Which of the following is not true for the order of a reaction?
When 10gram of methane is completely burnt in oxygen, the heat evolved is 560kJ. What is the heat of combustion (in kJ/mole) of methane?
The catalyst used for the polymerization of olefins is
When one Faraday current is passed which of the following would deposit one gram atomic weight of the metal?
A white salt is readily solubule in water and gives a colourless solution with a pH of about 9. The salt would be
The pH of a solution of hydrochloric acid is 4. The molarity of this solution is
Which one of the following can be considered as weak electrolyte ?
A solid cube has CsCl type structure. If the edge length of the unit cell is 404 pm, what is the distance between A⁺ and B⁻ ions?
Which of the following has maximum Schottky defect?
Select the correct statement regarding the aromatic nitrogen molecule
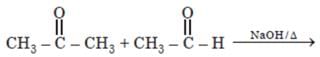
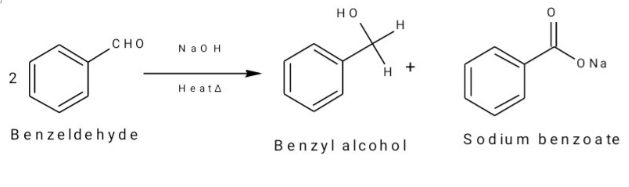
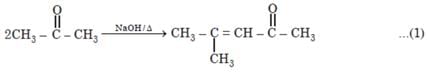
Number of possible self aldol condensation product on heating with NaOH?
Among the following the total number of elements which produce H2 gas with NaOH is Zn, Al, Sn, Pb, P, S
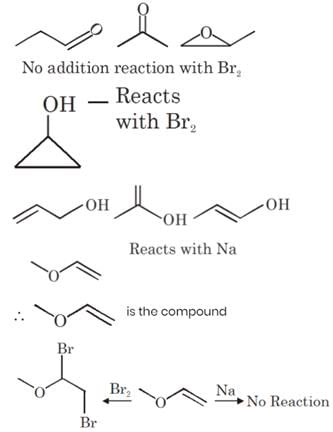
An organic compound P contains 62.07% carbon and 10.34% hydrogen and rest oxygen. Its vapour density is 29. This compound does not react with sodium metal, but its 2.9 g combines with X g of bromine (to give dibromo addition product). Find out value of (Y–X). (Where Y is total number of possible isomers of given organic compound P)
[Atomic mass : Br = 80]
How many of the following reagent/s will liberate at least one oxide of nitrogen as a product ?
(i) Ag + conc. HNO3
(ii) Sn + dil., HNO3 (20%)
(iii) Cu + conc. HNO3
(iv) C + conc. HNO3
(v) Zn + conc, HNO3
(vi) Zn + dil. HNO3 (20%)
(vii) P4 + conc. HNO3
(viii) S8 + conc. HNO3
(ix) Cu + dil. HNO3 (20%)
In white phosphorous (P4) if
x is total number of triangle
y is total number of plane of symmetry z is total number of P–P bond
then calculate value of (y + z)/x.