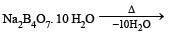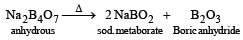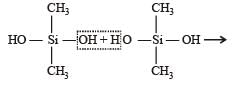31 Year NEET Previous Year Questions: The p-Block Elements (Group 13 & 14) - NEET MCQ
20 Questions MCQ Test - 31 Year NEET Previous Year Questions: The p-Block Elements (Group 13 & 14)
An example of a double salt is
The substance used as a smoke screen in warfare is[1989]
Glass is a [1991]
Which of the following types of forces bind together the carbon atoms in diamond ? [1992]
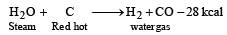
Water gas is produced by [1992]
Which of the following elements is extracted commercially by the electrolysis of an aqueous solution of its compound ? [1993]
Which of the following statements about H3BO3 is not correct ? [1994]
In graphite, electrons are [1994]
Glass reacts with HF to produce [2000]
In borax bead test which compound is formed?
Which one of the following statements about the zeolites is false ? [2004]
Al2O3 can be converted to anhydrous AlCl3 by heating [2006]
The straight chain polymer is formed by:[2009]
The tendency of BF3, BCl3 and BBr3 to behave as Lewis acid decreases in the sequence
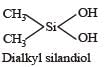
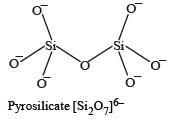
Name the type of the structure of silicate in which one oxygen atom of [SiO4]4– is shared ? [2011]
Aluminium is extracted from alumina (Al2O3) by electrolysis of a molten mixture of : [2012]
Which of the following structure is similar to graphite? [NEET 2013]
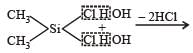
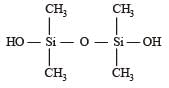
Which of these is not a monomer for a high molecular mass silicone polymer? [NEET 2013]
The basic structural unit of silicates is : [NEET 2013]
Which statement is wrong? [NEET Kar. 2013]