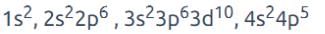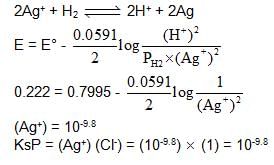AWES TGT Chemistry Mock Test - 3 - AWES TGT/PGT MCQ
30 Questions MCQ Test - AWES TGT Chemistry Mock Test - 3
Under NEP 2020, what is the target for the Gross Enrollment Ratio (GER) in higher education by 2035?
Institutional initiative for inclusive education can be evidenced when ______.
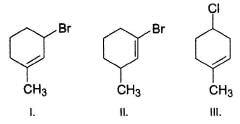
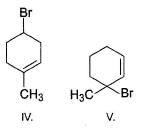
Rank the following molecules increasing in order of relative rate of SN1 solvolysis with methanol and heat.


MgO has the structure of NaCl. The coordination number of the ions in MgO is
During dehydration of alcohols to alkenes by heating with conc. H2SO4 the initiation step is-
[AIEEE-2003]
Yellow colour of NaCl crystals in sodium vapour is due to
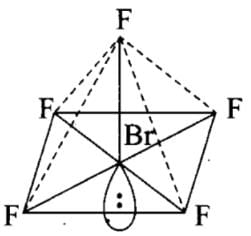
Which of the following has a square pyramidal shape?
In the modern periodic table, which period contains 32 elements?
Volume occupied by one molecule of water (density = 1 g cm-3) is
If Ksp for HgSO4 is 6.4 × 10-5, then solubility of this substance in mole per m3 is
Following cell has EMF 0.7995 V.
Pt | H2 (1 atm) | HNO3 (1M) || AgNO3 (1M) | Ag
If we add enough KCl to the Ag cell so that the final Cl- is 1M. Now the measured emf of the cell is 0.222 V.
The Ksp of AgCl would be :
The correct order of in creasing order of radiiions Br- , F-, O2- and S2- is as follow
In the third period of the periodic table the element having smallest size is
A hydrocarbon X is optically. X upon hydrogenation gives an optically inactive alkane Y. Which of the following pair of compounds can be X and Y respectively?
Enthalpies of formation of CO(g) , CO2 (g) , N2O (g) and N2O4 (g) are -110, - 393, 81 and 9.7 kJ mol-1. Thus, ΔrU for the reaction at 298 K is,
What is relative reactivity of secondary versus primary hydrogens in free radical bromination of n-butane if the ratio of 1-bromo to 2-bromobutane formed is 7 : 39?
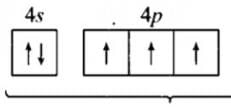
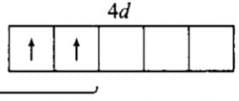
An element has configuration 4d55s2. The element belongs to
Which type of solids are held by weak dispersion forces?
Straight line graph for first order reaction is obtained between
Standard entropies of X2, Y2 and XY3 are given below the reaction
Q. At what temperature, reaction would be in equilibrium?
Which of the following angle corresponds to sp2 hybridisation?
Which one of the following statement(s) is/are incorrect for lanthanides?
Adsorption isobar is a curve showing variation of adsorption ______.
The golden yellow colour associated with NaCl to Bunsen flame can be explained on the basis of -