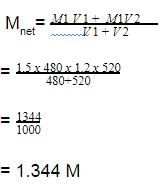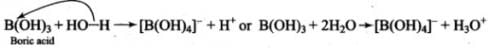AWES TGT Chemistry Mock Test - 4 - AWES TGT/PGT MCQ
30 Questions MCQ Test - AWES TGT Chemistry Mock Test - 4
Where is the 17th edition of the Joint Military Exercise SURYA KIRAN taking place?
How does NEP 2020 recommend promoting multilingualism among students?
‘Choice of challenge’ is a characteristic of which of the following?
An individual is in a transition state between childhood and adulthood he is an
Two solutions of a substance (non electrolyte) are mixed in the following manner. 480 ml of 1.5 M first solution + 520 mL of 1.2 M second solution. What is the molarity of the final mixture ?
[AIEEE-2005]
The catalytic activity of transition metals and their compounds is mainly due to
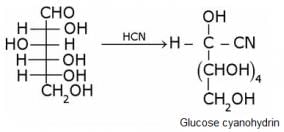
What does the following reaction shows about the structure of glucose?
The complex formation tendency of alkaline earth metals decreases down the group because -
Based on the following reaction,
It can be concluded that
Which of the following carbohydrates is called milk sugar?

Suitable reagent for following cenversion will be :
Stoichiometric compounds of dihydrogen are formed with
The number of cis-trans isomer possible for the following compound:
The standard reduction potential of a silver chloride electrode is 0.2 V and that of a silver electrode is 0.79 V. The maximum amount of AgCl that can dissolve in 106 L of a 0.1 M AgNO3 solution is
Which of the following amine will form stable diazonium salt at 273-283 K ?
10 dm3 of an ideal monoatomic gas at 27° C and 1.01 x 105 Nm-2 pressure are heated at constant pressure to 127°C. Thus entropy change is
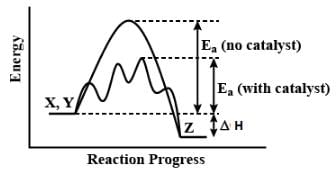
Consider the following reaction,
Q.
The major organic product is
Nitro compounds are reduced by iron scrap and hydrochloric acid to yield one of the following compounds.
Who was the scientist credited with devising the first periodic table similar to the one we use today?
Oxidation numbers of P in PO4−3, of S in SO42− and that of Cr in Cr2O72− are respectively,
10 ml of is mixed with 40 ml of
. The pH of the resulting solution is